Methods for disinfecting drinking water. Modern methods of water disinfection
Under the concepts of disinfection and disinfection of drinking water, it is customary to understand a number of complex measures that are aimed at the destruction of various viruses, bacteria, as well as the complete or partial removal of chemical impurities and other substances hazardous to the health of the body from the liquid. Water disinfection can be carried out both at special engineering and technical facilities on an industrial scale, and for local disinfection for quick consumption. In this article, we will consider the main methods of disinfecting drinking water and briefly describe their features.
Before disinfecting water, when choosing a means for disinfecting water, it should be understood that complete purification of water from all bacteria and minerals will make it unsuitable for human consumption. Therefore, choosing a method for disinfecting water, you need to be careful. There are several ways to influence microorganisms harmful to humans:
- Chemical methods of water disinfection (reagent);
- Physical methods (reagentless);
- Combined methods of exposure to microorganisms.
The chemical method includes the use of various coagulant reagents added to water for disinfection. This method also includes: chlorination, ozonation, the use of silver, silicon, sodium hypochlorite and other substances that can at least stop the reproduction of bacteria, and at most completely get rid of them.
Physical, reagent-free impact is made with the use of UV disinfection of water, electropulse and other methods.
Combined methods include both chemical and physical effects alternately. These methods are considered the most effective in disinfecting and cleaning from various impurities contained in water.
Disinfection of water by chemical methods

When using a chemical method of disinfection, it is extremely important to be able to determine or know the exact dosage, as well as the required time of exposure of the substance to water.
The required dose is determined both by trial disinfection and by calculation methods. Both an excess and a lack of a substance can make water unusable.
An example of incorrect dosage: A too small dose of ozone can kill only a part of the bacteria and, by forming special chemical compounds, will create an ideal environment for the reproduction of previously dormant bacteria.
To create a long-term effect of the destruction of microorganisms after disinfection, as a rule, the dose of the reagent is taken in excess. However, such an excess should not be dangerous to humans, since most reagents are quite toxic.
Water chlorination

Chlorine and its derivatives are still used in our country for water disinfection, despite the presence of many modern cleaning methods. This reagent shows good characteristics in terms of disinfection, even with minimal excess. So, at a residual chlorine concentration of 0.5 mg / l, the growth of pathogenic microorganisms in soda does not occur.
However, this reagent has a number of significant disadvantages: a high degree of toxicity, mutagenicity, and carcinogenicity. And even the subsequent purification of water with activated carbon is not able to completely remove the formed chlorine compounds. And if such waters go into the drain and enter the ground or river waters downstream, then the degree of adverse impact on nature is quite large.
The use of chlorine is largely due to the cheapness and availability of this reagent, and a high degree of effectiveness against pathogenic flora, algae growth, and a number of fungi. Under its influence, hydrogen sulfide is destroyed, iron and manganese are removed. It has the ability to bleach, making chlorine the main ingredient in most bleaches.
Chlorine dioxide has a greater effect on viruses and bacteria than ordinary chlorine, but pollutes the environment much less. But, this reagent is quite expensive and requires preparation directly at the place of use.
Chlorine forms so-called trihalomethanes (methane derivatives), which have a strong carcinogenic effect on the human body, leading to the growth of cancer cells. And when water is boiled, under the influence of high temperatures, dioxin is formed - a very strong poison.
As a result of a study by scientists from different countries, they showed that chlorine itself and its derivatives can cause all kinds of disorders and diseases of the internal organs of people from the gastrointestinal tract, cardiovascular system, liver, and kidneys. They destroy protein in the body, cause atherosclerosis, hypertension, all kinds of allergic manifestations. Detrimental to skin and hair.
Water ozonation

Ozonation, by decomposing ozone particles in water, forms atomic oxygen. As a result, the enzyme system of the microbial cell is destroyed. In addition, some of the compounds are oxidized, which causes a rather unpleasant odor, metal corrosion is accelerated (including kitchen utensils, plumbing systems, etc.). Therefore, when applying ozone, an accurate dosage is needed.
At the same time, this method is considered the best of the chemical ones, providing the fastest and safest water disinfection for the environment and humans.
This method requires special expensive equipment, high power consumption, as well as highly qualified service. All this makes this expensive method of disinfection applicable mainly in centralized water supply.
This is due to the fact that ozone is dangerous in the production process, explosive and toxic. Therefore, high-quality professional maintenance of such equipment or installations is extremely important.
In addition, recent studies have shown that ozonation alone is not enough for high-quality disinfection of water, since after its exposure, the decomposition of phenolic groups of humic substances begins. These substances contribute to the activation of previously "sleeping" microorganisms.
Water treated with ozone is transported in special containers made of certain types of plastic, asbestos cement, concrete, etc. Before putting such water through pipes and other metal containers, it is necessary to wait for the period of ozone decay.
Antiseptics, polymer reagents

Disinfection with polymeric reagents related to polymeric antiseptics is a separate method of water purification. Biolag is the best known of this class of reagents. Compared to ozone and chlorine, Biolag has a number of advantages:
- Does not harm health;
- Does not cause local irritation to the skin and mucous membranes;
- Does not cause allergic reactions;
- After purification, the water has no taste, smell and color;
- Does not spoil the fabric (swimming suits);
- Does not have a corrosive effect on metal surfaces;
- It has a long-term disinfection effect.
Other reagents

Disinfection with the help of reagents requires certain specific knowledge, since in this method the dosage tone and other calculations are important. A variety of heavy metal compounds are used, such as iodine, bromine, etc. This method is isolated separately as oligodynamic water disinfection.
When noble metals are used to purify water, for example, with the help of silver, there is not complete disinfection, but a temporary inhibition of the growth of the number of bacteria. In addition, with this method, it is extremely important to observe the dosage, since silver tends to accumulate in the human body and is very slowly and difficult to remove.
Other, more rare reagents, such as strong oxidizing agents (sodium hypochlorite), are used in cases where water values change frequently and are highly unstable. An example of the instability of water is the presence in it of organic substances, plankton. In terms of chemical and bactericidal properties, sodium hypochlorite is similar to chlorine, but it is not so harmful to the human body and the environment, it has a long bactericidal effect. This reagent is obtained by electrolysis of a 2-4% solution of sodium chloride (common salt) or mineralized waters.
The disadvantage of this method is that it takes much more energy to remove salt from water than chlorination. However, the undeniable advantage can be called safety for humans and the environment.
Disinfection of water by physical methods
Physical methods include exposure to ultrasound, disinfection of water with ultraviolet light and other methods. At the same time, preliminary filtration, coagulation of water is carried out in order to remove suspensions, helminth eggs and various microorganisms.
UV cleaning

For UV disinfection of water, the volume of liquid is calculated in order to calculate the required energy costs. To ensure efficiency, it is necessary to calculate the radiation power and exposure time, as well as take into account the degree of infection with bioorganisms (the number of microbes per 1 ml of water).
Determine the presence of BGKP (indicator bacteria belonging to the group of Escherichia coli). These bacteria are present in water contaminated with faecal matter and are extremely resistant to any disinfection processes. According to SanPiN 2.1.4.1074-01, the maximum allowable number of colipoma bacteria should not exceed 50 per 100 ml of liquid.
Ultraviolet disinfection has a more effective effect on various bioorganisms than chlorine. And with the ozonation method, in terms of cleaning efficiency, UV disinfection is approximately equal in efficiency.
Ultraviolet rays affect the enzyme systems of bacterial cells and cell metabolism. UV rays are able to destroy vegetative and spore bacteria, in the fight against which other methods are not very effective. At the same time, the taste, color and smell of water do not change, toxic substances are not formed, and an overdose of exposure is not possible.
However, this method has its drawback - the lack of aftereffect. At the same time, there is an indisputable plus - small installations for individual use at the cost of the process are on a par with chlorination, and are cheaper than ozonation. What makes this method applicable for use in private homes.
In order for this disinfecting method to remain effective, it is necessary to monitor the cleanliness of quartz lamps, which can accumulate mineral salt deposits. To solve this problem, food acid (vinegar, citric acid) is added to the water, and this solution is circulated through the system. In particular, vinegar copes very well with the problem of salt deposits. You can also apply mechanical cleaning of the surface of the lamps.
It should be noted that water treatment with ultraviolet radiation is carried out only after preliminary purification of water from substances capable of shielding the rays. The radiation wavelength can vary from 200 to 295 nm, but the most commonly used optimal value is 260 nm, at which the cell cytoplasm is actively destroyed. The service life of one UV lamp is about several thousand hours of continuous operation.
To date, ultraviolet radiation is the most effective method of disinfecting water.
Ultrasonic water treatment

Water treatment with ultrasound is based on the physical phenomenon of cavitation, that is, the ability to form voids that create a difference in pressure. Such dissonance leads to the death of bacteria as a result of rupture of cell membranes. This effect depends on the degree of intensity of sound vibrations. Ultrasonic cleaning units require qualified maintenance and are quite expensive.
Magnetostrictive or piezoelectric devices create a sound frequency of 48,000 Hz. At lower frequencies, the growth of bacteria not only does not stop, but also intensifies, so the accuracy of tuning and high-quality maintenance of such equipment is mandatory. Boiling water
Disinfection of water by boiling
Boiling is the most popular and widespread household method of water disinfection, during which (depending on the duration of the process) a huge number of pathogens die: bacteria, bacteriophages, viruses, etc. Gases dissolved in water are also eliminated, hardness (pH) decreases, while taste qualities practically do not change.
Integrated water purification methods

An integrated approach to cleaning includes both reagent methods and non-reagent methods. Water can be disinfected, for example, first with UV rays, and then, the disinfected volume of liquid, treated with chlorine. As a result, harmful microorganisms are eliminated, and secondary infection is excluded.
Combined methods save money on reagents and improve water quality.
Similarly, water can be disinfected first with ozone, and then chlorinated. In this case, the content of toxic compounds containing chlorine in the water is sharply reduced.
Filtration shows good results only when the disinfected volume of water passes through cells smaller than microorganisms. And given that most bacteria are about 1 micron in size, and viruses are even smaller in size, then in order to disinfect water, filter elements must have pores of 0.1-0.2 microns.
Purifier-type systems include several water purification systems at once with a fairly effective filtration system. Such equipment has a wide range of applications and is popular both at home and in office premises.
New water disinfection systems

Relatively new means of water disinfection: electropulse and electrochemical method. The bottom line is that water is passed through a diaphragm electrochemical reactor, which is separated by a metal-ceramic membrane. This membrane is capable of ultrafiltration to the cathode and anode region. After applying current to the anode and cathode chambers, alkaline and acidic solutions are formed, and, as a result, electrolytic formation, the so-called active chlorine. Such a water disinfectant can ensure the rapid death of almost all harmful microorganisms.
The method of electropulse action is capable of disinfecting with an electric charge, after which a superhigh pressure shock wave and light radiation arise. As a result, ozone is formed, which has a detrimental effect on microorganisms.
New cleaning methods are quite expensive and are not applicable in domestic household conditions due to the complexity of the ongoing processes and the need for constant qualified maintenance.
Note! Sanitary standards do not imply the complete destruction of all microorganisms contained in the water. It is required to remove and neutralize only bacteria, viruses and other inclusions that are dangerous to humans and can cause health problems. Completely sterile water is just as harmful to humans as contaminated with bacteria.
Before carrying out disinfection and making a choice of one or another cleaning method, it is necessary to first analyze the degree of water pollution: mineral, biological compounds and microorganisms. Based on the results of the analysis, the best option for high-quality disinfection and water purification is selected.
A person consumes about 2-3 liters of water per day - and this is only for drinking, not counting household needs. And it goes without saying that a liquid so important for our body must be safe and harmless - that is, it should not contain viruses and bacteria that can harm a person.

Moreover, the means for disinfecting water are relevant not only for tourists who need them in field conditions - such methods should also be used for your home. After all, water coming from a source (well or well) is hardly perfectly clean, which means it needs to be cleaned.
1 List of impurities that may be contained in water
Even crystal clear and transparent water can contain a huge number of microorganisms and impurities invisible to the human eye. Of course, not all of them harm our body. In particular, it does not tolerate:
- High content of manganese.
- High iron content (from 2-3 mg / l, however, an unpleasant taste appears already at a concentration of 0.3 mg / l).
- The presence of heavy metals - arsenic, copper, lead, mercury and so on. Moreover, even in a small amount they are harmful - because they accumulate in the body.
- The presence of nitrogen compounds (waste products of animals or humans, rotting plants or animal corpses).
- The presence of sodium in large quantities. The increased sodium content significantly spoils the taste of water.
- Bacteria belonging to the group of Escherichia coli.
In addition to the above impurities, water may contain calcium and magnesium. For the body, they do not pose a serious danger, however, at high concentrations, their presence leads to the appearance of scale - which means it harms the equipment.
2 Hiking methods of disinfection
Very often, methods of water disinfection are of interest to tourists and lovers of long hikes. In such cases, travelers usually take a small supply of drinking water with them, and replenish it from natural reservoirs.


This, of course, is interesting and exciting, but drinking water from a lake or river without first worrying about cleaning it is not a good idea.
First of all, for the reason that it may contain the aforementioned nitrogen compounds (rotting plants, corpses or animal waste products), which are very, very dangerous for the body and can lead to serious poisoning.
3 UV cleaning
Methods for water purification in stationary conditions are much more diverse. One of these tools is this. In this case, the neutralization of microbiological impurities occurs through radiation.
Such a water disinfectant is used both in cottages and in laboratories, hospitals, hotels, in industry - the lamp can be used almost everywhere.
The advantage of this method is that the lamp is highly likely to neutralize many of the most dangerous bacteria for the human body:
- coli;
- hepatitis;
- flu;
- salmonella;
- dysentery;
- cholera.
The aforementioned bacilli do not tolerate radiation doses less than 10 mJ/cm². At the same time, the lamp can provide a much larger limit - from 30 mJ / cm².
The water treatment plant, which is based on a lamp, works as follows: water enters the reaction chamber through the lower compartment of the housing. Passing near the source of radiation (actually - the lamp itself) and rushes up - to the outlet.


Everything - no other actions are required, that is, everything is extremely simple and fast. Such an apparatus for disinfecting water is good because it does not harm the human body and does not create a strong odor or taste (unlike chlorine).
Yes, and the lamp is also not too expensive - a compact installation of this type can even stand in the country.
The lamp has another advantage - an installation of this type can be easily mounted independently, without resorting to the services of specialists.
As for the service life - on average, the lamp is designed for 3-4 thousand hours of operation.
4 Ultrasonic cleaning
A bactericidal installation that neutralizes harmful microorganisms with ultrasound is more of an industrial rather than a household method. Its principle is based on the creation of ultrasonic waves (created by a special generator), which lead to a rupture of the cell membrane - and hence its death. For maximum efficiency of this method, the sound frequency should be about 48 thousand Hz.
One example of devices that purify liquids with ultrasound is the Lazur water disinfection apparatus. This is a modern bactericidal plant, which is used in industry and for large-scale water treatment. It is able to provide almost complete neutralization of any bacteria, converting them into neutral compounds.
Together with ultrasound (created by the generator), the Lazur unit also performs ultraviolet cleaning - combining methods and increasing the efficiency of the result. The procedure is performed simultaneously - both the lamp and the ultrasonic unit work inside the case.
5 Chemical cleaning methods
- the most common option for purifying any amount of water. It is, for example, used for swimming pools, for water treatment by municipal water utilities, water treatment plants.


The method itself is extremely simple: an active reagent is simply dosed into the water, which neutralizes microbes and bacteria. The following variations are used as the active substance:
- Bactericidal cleaning with chlorine.
- Cleaning with sodium hypochlorite.
- The use of bleach.
Alternatively, other chlorine compounds can be used. The most popular of the options is cleaning with sodium hypochlorite - "liquid chlorine".
The dosage of sodium hypochlorite in water is a cheap, but not the best solution:
- low efficiency;
- a large residual content of sodium hypochlorite in water - which is harmful to the body.
It turns out a vicious circle: too much sodium hypochlorite is impossible, because the water simply cannot be drunk. And too little - reduces the efficiency of water treatment.
The problem is usually solved by a complex method - in addition to sodium hypochlorite, water is additionally purified by any of the other methods. It can be either any of the ones mentioned above, or another option - water purification from chlorine itself.
So you can use sodium hypochlorite in high concentrations - the excess is then filtered, reducing the content of the substance to a safe level.
5.1 Hiking methods of water disinfection (video)
The most common water treatment processes are clarification and disinfection.
In addition, there are special ways to improve water quality:
- softening of water (elimination of water hardness cations);
- desalination of water (reduction of the total mineralization of water);
- deferrization of water (decrease in the concentration of iron salts in water);
- water degassing (removal of gases dissolved in water);
- neutralization of water (removal of toxic substances from water);
- water decontamination (water purification from radioactive contamination).
Disinfection is the final stage of the water purification process. The goal is to suppress the vital activity of pathogenic microbes contained in the water.
According to the method of impact on microorganisms, methods of water disinfection are divided into chemical, or reagent; physical, or reagentless, and combined. In the first case, the proper effect is achieved by introducing biologically active chemical compounds into the water; non-reagent methods of disinfection involve the treatment of water by physical influences, and in combined methods chemical and physical influences are used simultaneously.
Chemical methods of drinking water disinfection include its treatment with oxidizing agents: chlorine, ozone, etc., as well as heavy metal ions. To the physical - disinfection with ultraviolet rays, ultrasound, etc.
Chlorination is the most common chemical water disinfection method. This is due to the high efficiency, simplicity of the technological equipment used, the cheapness of the reagent used, and the relative ease of maintenance.
When chlorinating, bleach, chlorine and its derivatives are used, under the influence of which bacteria and viruses in the water die as a result of the oxidation of substances.
In addition to the main function of disinfection, due to its oxidizing properties and the preservative aftereffect, chlorine serves other purposes - controlling taste and smell, preventing algae growth, keeping filters clean, removing iron and manganese, destroying hydrogen sulfide, discoloration, etc.
According to experts, the use of gaseous chlorine leads to a potential risk to human health. This is primarily due to the possibility of the formation of trihalomethanes: chloroform, dichlorobromomethane, dibromochloromethane and bromoform. The formation of trihalomethanes is due to the interaction of active chlorine compounds with organic substances of natural origin. These methane derivatives have a pronounced carcinogenic effect, which contributes to the formation of cancer cells. When boiling chlorinated water, it produces the strongest poison - dioxin.
Studies confirm the relationship of chlorine and its by-products with the occurrence of diseases such as cancer of the digestive tract, liver, heart disorders, atherosclerosis, hypertension, and various types of allergies. Chlorine affects the skin and hair and also breaks down protein in the body.
One of the most promising methods for disinfecting natural water is the use of sodium hypochlorite (NaClO), obtained at the point of consumption by electrolysis of 2-4% sodium chloride solutions (common salt) or natural mineralized waters containing at least 50 mg/l of chloride ions. .
The oxidizing and bactericidal action of sodium hypochlorite is identical to dissolved chlorine, in addition, it has a prolonged bactericidal effect.
The main advantages of the sodium hypochlorite water disinfection technology are the safety of its use and a significant reduction in environmental impact compared to liquid chlorine.
Along with the advantages of water disinfection with sodium hypochlorite produced at the place of consumption, there are a number of disadvantages, first of all, an increased consumption of table salt, due to the low degree of its conversion (up to 10-20%). At the same time, the remaining 80-90% of salt in the form of ballast is introduced with a hypochlorite solution into the treated water, increasing its salt content. Reducing the salt concentration in the solution, undertaken for the sake of economy, increases the cost of electricity and the consumption of anode materials.
Some experts believe that replacing chlorine gas with sodium or calcium hypochlorite for water disinfection instead of molecular chlorine does not reduce, but greatly increases the likelihood of trihalomethane formation. The deterioration of water quality when using hypochlorite, in their opinion, is due to the fact that the process of formation of trihalomethanes is extended in time up to several hours, and their amount, ceteris paribus, is greater, the higher the pH (the value characterizing the concentration of hydrogen ions). Therefore, the most rational method for reducing the by-products of chlorination is to reduce the concentration of organic substances at the stages of water purification before chlorination.
Alternative methods of water disinfection associated with the use of silver are too expensive. An alternative method of water disinfection using ozone was proposed for chlorination, but it turned out that ozone also reacts with many substances in water - with phenol, and the resulting products are even more toxic than chlorophenolic ones. In addition, ozone is very unstable and quickly decomposes, so its bactericidal effect is short-lived.
Of the physical methods of disinfecting drinking water, the most widespread is the disinfection of water with ultraviolet rays, the bactericidal properties of which are due to the effect on cellular metabolism and, especially, on the enzyme systems of a bacterial cell. Ultraviolet rays destroy not only vegetative, but also spore forms of bacteria, and do not change the organoleptic properties of water. The main disadvantage of the method is the complete absence of aftereffect. In addition, this method requires more capital investment than chlorination.
The material was prepared on the basis of information from open sources
Water is a factor that directly affects the quality of human life. The mood of a person in the morning after washing depends on its color and smell, and the well-being and health of the body depends on the composition.
Water, being the basis of life, easily spreads infectious diseases. To prevent the transmission of pathogens through drinking water, decontamination and disinfection of the liquid is used. These processes allow to destroy fungi, bacteria, unpleasant aftertaste and color, which ensures the safety of drinking water.
Purification and disinfection of drinking water for supply to residential buildings is carried out at water treatment stations of centralized water supply. There are also methods and installations for local use - in the form of small water purification systems from a well or methods that allow you to purify water collected in a bottle.
Classification of water disinfection methods
To choose the right method of disinfection, analyze contaminated water. The number and type of microorganisms, the degree of side contamination are investigated. The volume of water to be treated and the economic factor are also determined.
Purified water is clear and colorless, does not smell and has no taste or taste. To achieve this effect, the following groups of methods are used:
- physical;
- chemical;
- combined.
Each group has its own distinctive features, but all methods one way or another allow you to remove pathogenic microorganisms from the water. You can get detailed information on equipment for water treatment and disinfection at the KVANTA + company in Tyumen.
The chemical method is the work with reagents added to water. Physical disinfection is carried out due to temperature or various radiations. Combined methods combine the work of these two groups.
The most efficient ways
The infectious safety of water is an important and urgent problem, which is why many methods have been invented to rid water of microorganisms. Disinfection methods continue to improve. They become more efficient and accessible. Currently, the following methods are considered the best:
- heat treatment using high temperatures;
- ultrasonic treatment;
- reagent methods;
- ultraviolet irradiation of the liquid;
- high-power electrical discharges.
Physical methods of water disinfection
Before them, the water must necessarily be cleaned from suspensions and impurities. For this, coagulation, sorption, flotation and filtration are used.
This type of method includes:
- ultrasound;
- ultraviolet;
- high temperatures;
- electricity.
UV disinfection
The disinfecting effect of ultraviolet radiation has been known for a very long time. Its work is similar to sunlight successfully destroying unadapted microorganisms outside the Earth's ozone layer. Ultraviolet affects cells by creating cross-links in DNA, as a result of which the cell loses the ability to divide and dies (Fig. 2).
 The installation consists of lamps placed in quartz cases. The lamps produce a study that instantly destroys microorganisms, and the covers do not allow the lamps to cool down. The quality of disinfection when using this method depends on the transparency of the water: the cleaner the incoming liquid, the farther the light propagates and the less the lamp becomes dirty. To do this, before disinfection, the water goes through other stages of purification, including mechanical filters. The tank through which the water flows is usually equipped with an agitator. Mixing the liquid layers allows the disinfection process to proceed more evenly.
The installation consists of lamps placed in quartz cases. The lamps produce a study that instantly destroys microorganisms, and the covers do not allow the lamps to cool down. The quality of disinfection when using this method depends on the transparency of the water: the cleaner the incoming liquid, the farther the light propagates and the less the lamp becomes dirty. To do this, before disinfection, the water goes through other stages of purification, including mechanical filters. The tank through which the water flows is usually equipped with an agitator. Mixing the liquid layers allows the disinfection process to proceed more evenly.
 The design of the UV disinfection unit
The design of the UV disinfection unit It is important to know that lamps and covers require regular maintenance: the structure must be disassembled and cleaned at least once a quarter.
Then the effectiveness of the process will not deteriorate due to the appearance of scale and other contaminants. The lamps themselves must be replaced once a year.
Ultrasonic disinfection units
The operation of such installations is based on cavitation. Due to the intense vibrations that water is subjected to due to high-frequency sound, numerous voids form in the liquid, as if it “boils”. An instantaneous pressure drop leads to rupture of cell membranes and death of microorganisms.
Equipment for ultrasonic water treatment is effective, but requires high costs and competent operation. It is important that the personnel know how to handle the device - its effectiveness depends on the quality of the equipment settings.
Thermal disinfection
This method is extremely common among the population and is actively used in everyday life. With the help of high temperature, that is, boiling, water is purified from almost all possible pathogenic organisms. In addition to this, water hardness is reduced and the content of dissolved gases is reduced. The taste of water remains the same. However, boiling has one drawback: water is considered safe for about a day, after which bacteria and viruses can again settle in it.
 Boiling water is a reliable and simple disinfection method.
Boiling water is a reliable and simple disinfection method. Electropulse disinfection
The technique is as follows: electric discharges entering the water create a shock wave, microorganisms fall under water hammer and die. This method does not require pre-cleaning and is effective even with increased turbidity. Not only vegetative, but also spore-forming bacteria die. The advantage is the long-term preservation of the effect (up to 4 months), and the disadvantage is the considerable cost and high energy consumption.
Chemical methods of water disinfection
They are based on the chemical reactions that take place between a contaminant or microorganism and a reagent added to the liquid.
In chemical disinfection, it is important to control the dose of the reagent.
It must be accurate. Lack of substance will not be able to fulfill its purpose. In addition, a small amount of reagent will lead to increased activity of viruses and bacteria.
To improve the performance of the chemical, it is added in excess. In this case, harmful microorganisms die, and the effect persists for a long time. The excess is calculated separately: if you add too much, the reagent will reach the consumer, and he will be poisoned.
Chlorination
Chlorine is widely distributed and used in water treatment in many countries of the world. It successfully copes with any volume of microbiological contamination. Chlorination leads to the death of most pathogenic organisms and is cheap and readily available. In addition, the use of chlorine and its compounds makes it possible to extract metals and hydrogen sulfide from water. Chlorination is used in urban drinking water supply systems. It is also used in swimming pools where a large number of people accumulate.
 However, this method has a number of disadvantages. Chlorine is extremely dangerous, causes cancer and cellular mutations, and is toxic. If excess chlorine does not disappear in the pipeline, but reaches the population, this can lead to serious health problems. The danger is especially strong during transitional periods (autumn and spring), when, due to an increase in surface water pollution, the dose of the reagent during water treatment is increased. Boiling such water will not help to avoid negative consequences, but on the contrary, chlorine will turn into dioxin, which is the strongest poison. In order to allow excess chlorine to evaporate, tap water is collected in large containers and left for a day in a well-ventilated area.
However, this method has a number of disadvantages. Chlorine is extremely dangerous, causes cancer and cellular mutations, and is toxic. If excess chlorine does not disappear in the pipeline, but reaches the population, this can lead to serious health problems. The danger is especially strong during transitional periods (autumn and spring), when, due to an increase in surface water pollution, the dose of the reagent during water treatment is increased. Boiling such water will not help to avoid negative consequences, but on the contrary, chlorine will turn into dioxin, which is the strongest poison. In order to allow excess chlorine to evaporate, tap water is collected in large containers and left for a day in a well-ventilated area.
Ozonation
Ozone has a strong oxidizing effect. It penetrates into the cell and destroys its walls, leading to the death of the bacterium. This substance is not only a strong antiseptic, but also discolors and deodorizes water, and oxidizes metals. Ozone works quickly and gets rid of almost all microorganisms in the water, overtaking chlorine in this characteristic.
Ozonation is considered the safest and most effective method, but it also has several disadvantages. An excess of ozone leads to corrosion of metal parts of equipment and pipelines, devices wear out and break down faster than usual. In addition, the latest research notes that ozonation causes the “awakening” of microorganisms that were in conditional hibernation.
 Scheme of the ozonation process
Scheme of the ozonation process The method is characterized by high cost of installation and high energy consumption. Highly qualified personnel are required to work with ozonizing equipment, because the gas is toxic and explosive. To release water to the population, it is necessary to wait out the period of ozone decay, otherwise people may suffer.
Disinfection with polymeric compounds
No harm to health, destruction of odors, tastes and colors, long duration of action - the listed advantages relate to disinfection using polymeric reagents. This type of substance is also called polymeric antiseptics. They do not cause corrosion and do not spoil the fabric, do not cause allergies and are distinguished by their effectiveness.
 Oligodynamia
Oligodynamia
It is based on the ability of noble metals (such as gold, silver and copper) to disinfect water.
The fact that these metals have an antiseptic effect has long been known. Copper and its alloys are often used in the field when it is necessary to individually disinfect a small volume of liquid.
For a more extensive effect of metals on microorganisms, ionizers are used. These are flow devices operating on the basis of a galvanic couple and electrophoresis.
Silver disinfection
This metal is considered to be one of the most ancient methods of water disinfection. In ancient times, it was widely believed that silver cures any disease. Now it is known that it negatively affects many microorganisms, but it is not known whether silver destroys protozoan bacteria.
This tool gives a visible effect in water purification. However, it negatively affects the human body when accumulated in it. No wonder silver has a high hazard class. Disinfection of water with silver ions is not considered a safe method, and therefore is practically not used in industry. Silver ionizers are used in isolated cases in everyday life for the treatment of small volumes of water.
 Compact household ionizer (silver) of water
Compact household ionizer (silver) of water Iodization and bromination
Iodine is widely known and has been used in medicine since ancient times. Scientists have repeatedly tried to use its disinfecting effect in water treatment, but its use leads to an unpleasant odor. Bromine does an excellent job with almost all known pathogens. But it has a significant drawback - high cost. Due to their disadvantages, these two substances are not used for the treatment of waste and drinking water.
Combined methods of water disinfection
Complex methods are based on a combination of physical and chemical methods to improve performance. An example is the combination of ultraviolet radiation and chlorination (sometimes chlorination is replaced by ozonation). UV lamps destroy microorganisms, and chlorine or ozone prevent their recurrence. In addition, oxidation and heavy metal processing are well combined. The oxidizing agent disinfects, and the metals prolong the bactericidal effect.
 Combination of UV disinfection and ultrasonic action
Combination of UV disinfection and ultrasonic action How to disinfect water at home
There are five ways to quickly disinfect a small amount of water:
- boiling;
- adding potassium permanganate;
- the use of disinfecting tablets;
- the use of herbs and flowers;
- infusion with silicon.
Potassium permanganate is added to water in the amount of 1-2 g per one bucket of water, after which the pollution precipitates.
Special tablets for the destruction of microorganisms are used to neutralize water from a well, well or spring. They are the most modern way, affordable, inexpensive and effective. Many tablets, such as the Aquatabs brand, can be used to clear large volumes of liquid.
If the water needs to be disinfected on a hike, you can use special herbs: St. John's wort, lingonberries, chamomile or celandine.
You can also use silicon: it is placed in water and left for a day.
Regulatory documentation in the field of drinking water safety
On the part of the state, water quality is strictly controlled with the help of regulations, rules and restrictions. The basis of legislative acts in the field of water resources protection and water quality control are two documents: the Federal Law "On the sanitary and epidemiological welfare of the population" and the Water Code.
The first law contains requirements for the quality of water supply sources from which water is supplied to residential buildings and for the needs of agriculture. The second document describes the norms for the use of water sources and instructions for ensuring their safety, and also defines penalties.
GOSTs
GOSTs describe the rules by which the quality control of waste and drinking water must be carried out. They contain methods for conducting analyzes in the field, and also allow you to divide water into groups. The most important of the GOSTs are presented in the table.
SNiPs
Building codes and regulations define the requirements for the construction of water treatment facilities, for the installation of various types of pipelines and water supply systems. Information is contained in SNiPs under the following numbers: SNiP 2.04.01-85, SNiP 3.05.01-85, SNiP 3.05.04-85.
SanPiNs
Sanitary and epidemiological rules and norms contain hygienic requirements for the quality of various groups of waters, for composition, for water intake facilities and the location of water intakes: SanPiN 2.1.4.559-96, SanPiN 4630-88, SanPiN 2.1.4.544-96, SanPiN 2.2.1 / 2.1 .1.984-00.
Thus, the effectiveness of disinfection of tap water is monitored with established regularity and in accordance with many rules and regulations. And a large number of different methods of fresh water disinfection allow you to choose the best option for any conditions. What makes properly purified and treated water safe for human consumption.
Disinfection of drinking water serves to create a reliable barrier to the transmission of pathogens of infectious diseases by water. Methods of water disinfection are aimed at the destruction of pathogenic and opportunistic microorganisms, which ensures the epidemic safety of water.
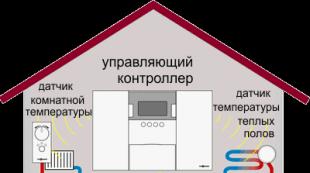
Water is disinfected at the final stage of purification after clarification and discoloration before entering clean water tanks, which simultaneously act as contact chambers. Reagent (chemical) and non-reagent (physical) methods are used for water disinfection. Reagent methods are based on the introduction of strong oxidizing agents into water (chlorination, ozonation, manganation, water treatment with iodine), heavy metal ions and silver ions. The non-reagent ones include heat treatment, ultraviolet irradiation, sonication, y-irradiation, treatment with microwave current. The method is chosen depending on the quantity and quality of the source water, the methods of its preliminary treatment, the requirements for the reliability of disinfection, taking into account technical and economic indicators, the conditions for the supply of reagents, the availability of transport, and the possibility of automating the process.
Disinfection of water with chlorine and its compounds. To date, the most common method of water disinfection at waterworks is chlorination. Among chlorine-containing compounds, given certain hygienic and technical advantages, liquid chlorine is most often used. It is also possible to use bleach, calcium and sodium hypochlorite, chlorine dioxide, chloramines, etc.
*For use in the practice of domestic and drinking water supply, only fluorine-containing compounds that have passed hygienic testing and are included in the "List of materials and reagents permitted by the Main Sanitary and Epidemiological Directorate of the Ministry of Health of the USSR for use in the practice of domestic and drinking water supply (No. 3235-85)" .*
For the first time in the practice of water treatment, chlorine was used long before the discovery of microbes by L. Pasteur, R. Koch's proof of the etiological significance of pathogenic microorganisms in the development of infectious diseases, T. Escherich's final awareness of the microbiological essence of water epidemics and the bactericidal properties of chlorine. It was used to deodorize water, which had an unpleasant "septic" smell. Chlorine proved to be a very effective deodorant and, in addition, after treating water with chlorine, people were much less likely to be diagnosed with intestinal infections. With the beginning of water chlorination, epidemics of typhoid and cholera stopped in many European countries. It has been suggested that the bad smell and taste of the water, which the chlorine effectively eliminated, was the cause of the illnesses. Only with time proved the microbial etiology of water epidemics of intestinal infections and recognized the role of chlorine as a disinfecting agent.
To chlorinate water, liquid chlorine is used, which is stored under pressure in special containers (cylinders), or substances containing active chlorine.
Chlorination of water with liquid chlorine. Chlorine (C12) at normal atmospheric pressure is a greenish-yellow gas, which in 1.5-
2.5 times heavier than air, with a sharp and unpleasant odor, dissolves well in water, easily liquefies when pressure increases. The atomic weight of chlorine is 35.453, the molecular weight is 70.906 g/mol. Chlorine can be in three states of aggregation: solid, liquid and gaseous.
Chlorine is delivered to waterworks for water disinfection in liquid form in pressurized cylinders. Chlorination is carried out using chlorinators. They prepare a solution of chlorine, which is injected directly into the pipeline, through which water enters the RCHV. L.A. chlorinators are used. Kulsky (Fig. 20), vacuum chlorinators LONII-100, Zh-10, LK-12, KhV-11. Schematic diagram of the LONII-100 chlorinator is shown in fig. 21.
When the cylinder is connected to the chlorinator, liquid chlorine evaporates. Gaseous chlorine is purified in a cylinder and on a filter, and after reducing its pressure with a reducer to 0.001-0.02 MPa, it is mixed in a mixer with water. From the mixer concentrated
Rice. 21. Technological scheme of a typical chlorination plant for 3 kg/h: 1 - platform scales; 2 - risers with cylinders; 3 - dirt trap; 4 - LONII-100 chlorinators; 5 - ejectors
The solution is sucked in by the ejector and fed into the pipeline. Chlorinators of the LK type, whose design is simpler, and the accuracy is lower, are used for high-capacity stations. These chlorinators do not require preliminary purification of chlorine, are not so accurate in dosing, but can supply chlorine water to a height of 20-30 m. After the ejector from LONI-100, the pressure is only 1-2 m. During the dissolution of chlorine in water, its hydrolysis occurs with the formation of chloride (hydrochloric) and hypochlorite (or hypochlorous) acids:
C12 + H20 ^ HCl + HC10.
Hypochlorous acid HC10 is a weak, monobasic, unstable acid that readily dissociates to form hypochlorite ion (CH~):
NSJ ^ H+ + SU".
The degree of dissociation of hypochlorous acid depends on the pH of the water. At pH
In addition, hypochlorous acid decomposes to form atomic oxygen, which is also a strong oxidizing agent:
HCS It HCl + O".
* Active chlorine is one that is capable of releasing an equivalent amount of iodine from aqueous solutions of potassium iodide at pH 4. There are free (molecular chlorine, hypochlorous acid, hypochlorite ion) and bound (chlorine, which is part of organic and inorganic mono- and dichloramines) active chlorine. *
Previously, it was believed that it was this atomic oxygen that had a bactericidal effect. Today it has been proven that the disinfecting effect of liquid chlorine, as well as bleach, calcium and sodium hypochlorites, the two-tertiary salt of calcium hypochlorite, is due to oxidizing agents that are formed in water when chlorine-containing compounds are dissolved, and first of all, by the action of hypochlorite acid, and then by the hypochlorite anion and finally atomic oxygen.
Chlorination of water with hypochlorites (salts of hypochlorous acid) is carried out at low power waterworks. Hypochlorites are also used for long-term disinfection of water in mine wells using ceramic cartridges, for disinfection of water in the field, including the use of fabric-carbon filters, etc.
Calcium hypochlorite Ca(OC1)2 is used to disinfect drinking water. In the process of its dissolution in water, hydrolysis occurs with the formation of hypochlorous acid and its further dissociation:
Ca (OS1) 2 + 2H20 \u003d Ca (OH) 2 + 2 HNS,
Neyu -?. n+ + cicr.
Depending on the method of calcium production, hypochlorite can contain from 57-60% to 75-85% active chlorine. Together with pure hypochlorite, a mixture of calcium hypochlorite with other salts (NaCl, CaCl2) is used to disinfect water. Such mixtures contain up to 60-75% pure hypochlorite.
At stations with an active chlorine consumption of up to 50 kg / day, sodium hypochlorite (NaCIO 5H20) can be used to disinfect water. This crystalline hydrate is obtained from a solution of sodium chloride (NaCl) by an electrolytic method.
Sodium chloride in water dissociates with the formation of sodium cation and chloride anion:
NaCl ^ Na+ + SG
During electrolysis at the anode, chlorine ions are discharged and molecular chlorine is formed:
2SG -» C12 + 2e.
The resulting chlorine dissolves in the electrolyte:
C12 + H2O ^ HC1 + HSC,
C12 + OH-^C1 + NSO.
Discharge of water molecules occurs at the cathode:
H20 + e -> OH- + H+.
Hydrogen atoms after recombination into molecular hydrogen are released from solution in the form of a gas. Hydroxyl anions OH ", remaining in the water, react with sodium cations Na +, resulting in the formation of NaOH. Sodium hydroxide interacts with hypochlorous acid to form sodium hypochlorite:
NaOH + HC10 -> NaOCI + H20.
Rice. 22. Technological scheme of electrolytic production of sodium hypochlorite: 1 - solution tank; 2 - pump; 3 - distribution tee; 4 - working tank; 5 - dispenser; 6 - cell with graphite electrodes; 7 - sodium hypochlorite storage tank; 8 - exhaust ventilation umbrella
Sodium hypochlorite dissociates to a large extent with the formation of SJ, which has a high antimicrobial activity:
NaCIO ^ Na+ + CIO",
Xu- + n+;^nsyu.
Electrolysis plants are divided into flow and batch. They include electrolyzers, various types of tanks. The schematic diagram of the batch plant is shown in fig. 22. A 10% concentration sodium chloride solution is fed into a constant level tank, from where it flows out at a constant rate. After filling the dosing tank, the siphon works and drains a certain volume of solution into the electrolyzer. Under the influence of electric current, sodium hypochlorite is formed in the electrolyzer. New portions of the salt solution push sodium hypochlorite into the supply tank, from which it is dosed by a dosing pump. The storage tank must hold a volume of sodium hypochlorite for at least 12 hours.
The advantage of obtaining sodium hypochlorite by the electrolytic method at the point of use is that there is no need to transport and store toxic liquefied chlorine. Among the disadvantages are significant energy costs.
Disinfection of water by direct electrolysis. The method consists in the direct electrolysis of fresh water, in which the natural content of chlorides is not lower than 20 mg/l, and the hardness is not higher than 7 mg-eq/l. They are used at waterworks with a capacity of up to 5000 m3 / day. Due to direct electrolysis at the anode, the chloride ions in the water are discharged and molecular chlorine is formed, which is hydrolyzed to form hypochlorous acid:
2CH ^ C12 + 2e, C12 + H2O^HC1 + HCO.
During electrolysis treatment of water with a pH in the range of 6-9, the main disinfecting agents are hypochlorous (hypochlorite) acid HCl, hypochlorite anion C10 ~ and monochloramines NH2C1, which are formed due to the reaction between HCl and ammonium salts contained in natural water. At the same time, during the treatment of water by the electrolytic method, the microorganisms are affected by the electric field in which they are located, which enhances the bactericidal effect.
Disinfection of water with bleach is used at small waterworks (with a capacity of up to 3000 m3 / day), after preparing the solution. Chlorine lime is also filled with ceramic cartridges for water disinfection in mine wells or local water supply systems.
Bleach is a white powder with a strong chlorine odor and strong oxidizing properties. It is a mixture of calcium hypochlorite and calcium chloride. Get bleach from limestone. Calcium carbonate at a temperature of 700 ° C decomposes with the formation of quicklime (calcium oxide), which, after interaction with water, turns into slaked lime (calcium hydroxide). When chlorine reacts with slaked lime, bleach is formed:
CaCO3 ^ CaO + CO2,
CaO + H20 \u003d Ca (OH) 2,
2Ca(OH)2 + 2C12 = Ca(OC1)2 + CaC12+ 2H20 or
2Ca(OH)2 + 2C12= 2CaOC12 + 2H20.
The main component of bleach is expressed by the formula:
The technical product contains no more than 35% active chlorine. During storage, bleach partially decomposes. The same happens with calcium hypochlorite. Light, humidity and high temperature accelerate the loss of active chlorine. Bleach loses approximately 3-4% of available chlorine per month due to hydrolysis reactions and decomposition in the light. In a damp room, bleach decomposes, forming hypochlorous acid:
2CaOC12 + CO2 + H20 = CaCO3 + CaC12 + 2HCO.
Therefore, before using bleach and calcium hypochlorite, their activity is checked - the content of active chlorine expressed as a percentage in a chlorine-containing preparation.
The bactericidal action of bleach, as well as hypochlorites, is due to the group (OSG), which forms hypochlorous acid in the aquatic environment:
2CaOC12 + 2H20 -> CaC12 + Ca(OH)2 + 2HC10.
Chlorine dioxide (ClOJ is a yellow-green gas, easily soluble in water (at a temperature of 4 ° C, 20 volumes of CO2 gas dissolve in 1 volume of water). It does not hydrolyze. It is advisable to use it if the characteristics of natural water are unfavorable for effective disinfection chlorine, for example, at high pH values or in the presence of ammonia.However, the production of chlorine dioxide is a complex process that requires special equipment, qualified personnel, additional financial costs.In addition, chlorine dioxide is explosive, which requires strict adherence to safety requirements. the use of chlorine dioxide for water disinfection in domestic and drinking water pipelines.
Chlorine-containing preparations also include chloramines (inorganic and organic), which are used to a limited extent in the practice of water treatment, but are used as disinfecting agents during disinfection measures, in particular in medical institutions. Inorganic chloramines (monochloramines NH2C1 and dichloramines NHC12) are formed by the interaction of chlorine with ammonia or ammonium salts:
NH3 + CI2 = NH2CI + HCI,
NH2CI + CI2 = NHCI2 + HCl.
Together with inorganic chlorine compounds, organic chloramines (RNHC1, RNC12) are also used for disinfection. They are obtained in the process of interaction of bleach with amines or their salts. In this case, one or two hydrogen atoms of the amine group are replaced by chlorine. Different chloramines contain 25-30% active chlorine.
The process of water disinfection with chlorine-containing preparations occurs in several stages:
1. Hydrolysis of chlorine and chlorine-containing preparations:
C12 + H20 = HCl + HC10;
Ca (OC1) 2 + 2H20 \u003d Ca (OH) 2 + 2HC10;
2CaOC12 + 2H20 \u003d Ca (OH) 2 + CaC12 + 2HC10.
2. Dissociation of hypochlorous acid.
At pH ~ 7.0 HC10 dissociates: HC10
3. Diffusion of the HC10 molecule and the CO ion into the bacterial cell.
4. Interaction of a disinfecting agent with enzymes of microorganisms, which are oxidized by hypochlorous acid and hypochlorite ion.
Active chlorine (NSO and SU") first diffuses into the bacterial cell, and then reacts with enzymes. The greatest bactericidal and virucidal effect is exerted by undissociated hypochlorous acid (NSO). The rate of the water disinfection process is determined by the kinetics of chlorine diffusion into the bacterial cell and the kinetics of cell death as a result of metabolic disorders.With an increase in the concentration of chlorine in water, its temperature and with the transition of chlorine to the non-dissociated form of easily diffusible hypochlorous acid, the overall rate of the disinfection process increases.
The mechanism of the bactericidal action of chlorine consists in the oxidation of organic compounds of a bacterial cell: coagulation and damage to its membrane, inhibition and denaturation of enzymes that provide metabolism and energy. The most damaged are thiol enzymes containing SH-groups, which are oxidized by hypochlorous acid and hypochlorite ion. Among the thiol enzymes, the group of dehydrogenases, which provide respiration and energy metabolism of the bacterial cell, is most actively inhibited1. Under the influence of hypochlorous acid and hypochlorite ion, dehydrogenases of glucose, ethyl alcohol, glycerol, succinic, glutamic, lactic, pyruvic acids, formaldehyde, etc. are inhibited. Inhibition of dehydrogenases leads to inhibition of oxidation processes at the initial stages. The consequence of this is both the inhibition of the processes of reproduction of bacteria (bacteriostatic action) and their death (bactericidal action).
The mechanism of action of active chlorine on viruses consists of two phases. First, hypochlorous acid and hypochlorite ion are adsorbed on the virus envelope and penetrate through it, and then they inactivate the RNA or DNA of the virus.
As the pH value increases, the bactericidal activity of chlorine in water decreases. For example, to reduce the number of bacteria in water by 99% at a dose of free chlorine of 0.1 mg/l, the contact time increases from 6 to 180 minutes with an increase in pH from 6 to 11, respectively. Therefore, it is advisable to disinfect water with chlorine at low pH values, i.e. before the introduction of alkaline reagents.
The presence in water of organic compounds capable of oxidation, inorganic reducing agents, as well as colloidal and suspended substances enveloping microorganisms, slows down the process of water disinfection.
The interaction of chlorine with water components is a complex and multi-stage process. Small doses of chlorine are completely bound by organic substances, inorganic reducing agents, suspended particles, humic substances and water microorganisms. For a reliable disinfecting effect of water after its chlorination, it is necessary to determine the residual concentrations of free or combined active chlorine.
* Energy metabolism in bacteria occurs in mesosomes - analogues of mitochondria. *
Rice. 23. Graph of the dependence of the magnitude and type of residual chlorine on the injected dose of chlorine
On fig. 23 shows the relationship between the dose of introduced chlorine and residual chlorine in the presence of ammonia or ammonium salts in the water. When chlorinating water that does not contain ammonia or other nitrogen-containing compounds, "with an increase in the amount of chlorine introduced into the water, the content of residual free chlorine in it increases. But the picture changes if there is ammonia, ammonium salts and other nitrogen-containing compounds in the water, which are an integral part of natural water or artificially introduced into it.At the same time, chlorine and chlorine agents interact with the ammonia present in the water, ammonium and organic salts containing amino groups.This leads to the formation of mono- and dichloramines, as well as extremely unstable trichloramines:
NH3 + H20 = NH4OH;
C12 + H20 = HC10 + HCl;
NSO + NH4OH = NH2C1 + H20;
NSC + NH2C1 = NHC12 + H20;
NSJ + NHC12 = NC13 + H20.
Chloramines are bound active chlorine, which has a bactericidal effect, which is 25-100 times less than that of free chlorine. In addition, the ratio between mono- and dichloramines changes depending on the pH of the water (Fig. 24). At low pH values (5-6.5), dichloramines are predominantly formed, and at high pH values (greater than 7.5), monochloramines are formed, the bactericidal effect of which is 3-5 times weaker than that of dichloramines. The bactericidal activity of inorganic chloramines is 8-10 times higher than that of chlorine derivatives of organic amines and imines. When adding low doses of chlorine to water at a molar ratio of C12: NH *
*Ammonia-free water does not exist in nature. It can only be prepared in the laboratory from distilled water.*
residual amine-bound chlorine accumulates. With an increase in the dose of chlorine, more chloramines are formed and the concentration of residual combined chlorine rises to a maximum (point A).
With a further increase in the dose of chlorine, the molar ratio of the introduced chlorine and the NH* ion contained in water becomes greater than one. In this case, mono-, di- and, especially, trichloramines are oxidized by excess chlorine in accordance with the following reactions:
NHC12 + NH2C1 + NSO -> N20 + 4HC1;
NHC12 + H20 -> NH(OH)Cl + HCl;
NH(OH)Cl + 2HC10 -> HN03 + ZHC1;
NHC12 + HCIO -> NC13 + H20;
4NH2C1 + 3C12 + H20 = N2 + N20 + 10HC1;
IONCI3 + CI2 + 16H20 = N2 + 8N02 + 32HCI.
At a molar ratio of Cl2: NH \ up to 2 (10 mg Cl2 per 1 mg N2 in the form of NH \), due to the oxidation of chloramines with excess chlorine, the amount of residual combined chlorine in water decreases sharply (section III) to a minimum point (point B), which is called the point fracture. Graphically, it looks like a deep dip in the residual chlorine curve (see Fig. 23).
With a further increase in the dose of chlorine after the turning point, the concentration of residual chlorine in the water again begins to gradually increase (segment IV on the curve). This chlorine is not associated with chloramines, is called free residual (active) chlorine and has the highest bactericidal activity. It acts on bacteria and viruses like active chlorine in the absence of ammonia and ammonium compounds in water.
According to research data, water can be disinfected with two doses of chlorine: before and after the fracture. However, when chlorinating with a pre-fracture dose, water is disinfected due to the action of chloramines, and when chlorinating with a post-fracture dose, free chlorine is used.
During water disinfection, the added chlorine is consumed both for interaction with microbial cells and viruses, and for the oxidation of organic and mineral compounds (urea, uric acid, creatinine, ammonia, humic substances, ferrous salts, ammonium salts, carbamates, etc.). ), which are contained in water in a suspended and dissolved state. The amount of chlorine absorbed by water impurities (organic substances, inorganic reducing agents, suspended particles, humic substances and microorganisms) is called the chlorine absorption of water (segment I on the curve). Since natural waters have a different composition, the value of chlorine absorption is not the same for them. Thus, chlorine absorption is the amount of active chlorine that is absorbed by suspended particles and is spent on the oxidation of bacteria, organic and inorganic compounds contained in 1 liter of water.
You can count on successful disinfection of water only if there is a certain excess of chlorine in relation to the amount that is absorbed by bacteria and various compounds contained in the water. An effective dose of active chlorine is equal to the total amount of absorbed and residual chlorine. The presence of residual chlorine (or, as it is also called, excess) in water is associated with the idea of the effectiveness of water disinfection.
When water is chlorinated with liquid chlorine, calcium and sodium hypochlorites, bleach, 30-minute contact provides a reliable disinfecting effect at a residual chlorine concentration of at least 0.3 mg/l. But during chlorination with preammonization, the contact should be for 1-2 hours, and the disinfection efficiency will be guaranteed if there is residual combined chlorine at a concentration of at least 0.8 mg/l.
Chlorine and chlorine-containing compounds significantly affect the organoleptic properties of drinking water (smell, taste), and in certain concentrations irritate the mucous membranes of the oral cavity and stomach. The limiting concentration of residual chlorine, at which drinking water does not acquire a chlorine smell and taste, is set for free chlorine at the level of 0.5 mg/l, and for bound chlorine - 1.2 mg/l. According to toxicological signs, the maximum concentration of active chlorine in drinking water is 2.5 mg/l.
Therefore, to disinfect water, it is necessary to add such an amount of a chlorine-containing preparation so that after treatment the water contains 0.3-0.5 mg/l of residual free or 0.8-1.2 mg/l of residual combined chlorine. Such an excess of active chlorine does not impair the taste of water, does not harm health, but guarantees its reliable disinfection.
Thus, for effective disinfection, a dose of active chlorine is added to water, equal to the sum of chlorine absorption and residual active chlorine. This dose is called the chlorine requirement of the water.
The chlorine demand of water is the amount of active chlorine (in milligrams) necessary for the effective disinfection of 1 liter of water and ensuring the content of residual free chlorine in the range of 0.3-0.5 mg / l after 30 minutes of contact with water, or the amount of residual combined chlorine within 0.8-1.2 mg after 60 minutes of contact. Residual content
*The maximum concentration of chlorine dioxide in drinking water is not higher than 0.5 mg/l, the limiting indicator of water action is organoleptic.*
Active chlorine is controlled after clean water tanks before being supplied to the water supply network. Since the chlorine absorption of water depends on its composition and is not the same for water from different sources, in each case the chlorine demand is determined experimentally by test chlorination. Approximately, the chlorine demand for clarified and discolored coagulation, settling and filtration of river water ranges from 2-3 mg / l (sometimes up to 5 mg / l), water of underground interstratal waters - within 0.7-1 mg / l.
Factors affecting the process of water chlorination are associated with: 1) biological characteristics of microorganisms; 2) bactericidal properties of chlorine-containing preparations; 3) the state of the aquatic environment; 4) with the conditions under which disinfection is carried out.
It is known that spore cultures are many times more resistant than vegetative forms to the action of disinfectants. Enteroviruses are more resistant than intestinal bacteria. Saprophytic microorganisms are more resistant than pathogenic ones. At the same time, among pathogenic microorganisms, the most sensitive to chlorine are the causative agents of typhoid fever, dysentery, and cholera. The causative agent of paratyphoid B is more resistant to the action of chlorine. In addition, the higher the initial contamination of water with microorganisms, the lower the efficiency of disinfection under the same conditions.
The bactericidal activity of chlorine and its compounds is related to the value of its redox potential. The redox potential increases at the same concentrations in the series: chloramine -\u003e bleach -\u003e chlorine -\u003e chlorine dioxide.
The effectiveness of chlorination depends on the properties and composition of the aquatic environment, namely: on the content of suspended solids and colloidal compounds, the concentration of dissolved organic compounds and inorganic reducing agents, the pH of the water, and its temperature.
Suspended substances and colloids prevent the impact of the disinfecting agent on microorganisms located in the thickness of the particle, absorb active chlorine due to adsorption and chemical binding. The effect on the efficiency of chlorination of organic compounds dissolved in water depends both on their composition and on the properties of chlorine-containing preparations. So, nitrogen-containing compounds of animal origin (proteins, amino acids, amines, urea) actively bind chlorine. Compounds that do not contain nitrogen (fats, carbohydrates) react less strongly with chlorine. Since the presence of suspended solids, humic and other organic compounds in water reduces the effect of chlorination, for reliable disinfection, turbid and high-colored waters are preliminarily clarified and discolored.
When the water temperature drops to 0-4 °C, the bactericidal effect of chlorine decreases. This dependence is especially noticeable in experiments with high initial contamination of water and in the case of chlorination with low doses of chlorine. In the practice of waterworks, if the contamination of the source water meets the requirements of State Standard 2761-84 "Sources of centralized domestic drinking water supply. Hygienic, technical requirements and quality control", a decrease in temperature does not noticeably affect the effectiveness of disinfection.
The mechanism of the influence of the pH of water on its disinfection with chlorine is associated with the features of the dissociation of hypochlorous acid: in an acidic environment, the equilibrium shifts towards the molecular form, in an alkaline environment - ionic. Hypochlorous acid in its undissociated molecular form penetrates better through membranes into the middle of the bacterial cell than hydrated hypochlorite ions. Therefore, in an acidic environment, the process of water disinfection is accelerated.
The bactericidal effect of chlorination is significantly affected by the dose of the reagent and the duration of contact: the bactericidal effect increases with an increase in the dose and an increase in the duration of action of active chlorine.
Water chlorination methods. There are several methods of chlorination. water treatment, taking into account the nature of residual chlorine, the choice of which is determined by the characteristics of the composition of the treated water. Among them: 1) chlorination with post-fracture doses; 2) conventional chlorination or chlorination according to chlorine demand; 3) superchlorination; 4) chlorination with preammonization. In the first three options, water is disinfected with free active chlorine. During chlorination with preammonization, the bactericidal effect is due to the action of chloramines, i.e., bound active chlorine. In addition, combined methods of chlorination are used.
Chlorination with post-fracture doses provides that after 30 minutes of contact, free active chlorine will be present in the water. The dose of chlorine is selected in such a way that it is slightly higher than the dose at which a break is formed on the curve of residual chlorine, i.e., in the range IV (see Fig. 23). The dose selected in this way causes the appearance of residual free chlorine in the water in the smallest amount. This method is characterized by careful dose selection. It gives a stable and reliable bactericidal effect, prevents the appearance of odors in the water.
Conventional chlorination (chlorination according to chlorine demand) is the most common method of disinfecting drinking water in a centralized domestic drinking water supply. Chlorination according to chlorine demand is carried out with such a post-breaking dose, which after 30 minutes of contact ensures the presence of residual free chlorine in the water in the range of 0.3-0.5 mg / l.
Since natural waters differ significantly in composition and therefore have different chlorine absorption, chlorine demand is determined experimentally by experimental chlorination of water to be disinfected. In addition to the correct choice of the dose of chlorine, a prerequisite for effective water disinfection is thorough mixing and exposure time, i.e., the contact time of chlorine with water (at least 30 minutes).
As a rule, at waterworks chlorination according to chlorine demand is carried out after clarification and discoloration of water. The chlorine demand of such water ranges from 1-5 mg/l. The optimal dose of chlorine is introduced into the water immediately after filtration before the RFW.
Based on the chlorine demand, double chlorination can also be carried out, in which the first time chlorine is fed into the mixer before the reaction chamber, and the second time after the filters. In this case, the experimentally determined optimal dose of chlorine is not changed. Chlorine, when introduced into the mixer in front of the reaction chamber, improves coagulation and water discoloration, which makes it possible to reduce the dose of coagulant. In addition, it inhibits the growth of microflora that contaminates the sand on the filters. The total cost of chlorine with double chlorination practically does not increase and remains almost the same as with single chlorination.
Double chlorination deserves widespread use. It should be consulted in cases where the pollution of river water is relatively high or subject to frequent fluctuations. Double chlorination increases the sanitary reliability of water disinfection.
Superchlorination (rechlorination) is a method of water disinfection, which uses high doses of active chlorine (5-20 mg/l). These doses are actually post-fracture. In addition, they significantly exceed the chlorine demand of natural water and determine the presence in it of high (over 0.5 mg/l) concentrations of residual free chlorine. Therefore, the superchlorination method does not require a preliminary determination of the chlorine demand of water and a careful selection of the dose of active chlorine, however, after disinfection, it is necessary to remove excess free chlorine.
Superchlorination is used in a special epidemiological situation, when it is impossible to determine the chlorine demand of water and ensure sufficient contact time of chlorine with water, as well as to prevent and combat odors in water. This method is convenient in military field conditions, in emergency situations.
Superchlorination effectively provides reliable disinfection even of turbid water. High doses of active chlorine kill pathogens that are resistant to disinfectants, such as Burnett's rickettsiae, amoeba cysts, mycobacterium tuberculosis and viruses. But even such doses of chlorine cannot reliably disinfect water from anthrax spores and helminth eggs.
During superchlorination, residual free chlorine in disinfected water significantly exceeds 0.5 mg / l, which makes the water unsuitable for drinking due to the deterioration of its organoleptic properties (strong smell of chlorine). Therefore, there is a need to free it from excess chlorine. This process is called dechlorination. If there is little excess residual chlorine, it can be removed by aeration. In other cases, water is purified by filtering through a layer of activated carbon or using chemical methods, such as treating sodium with hyposulfite (thiosulfate), sodium bisulfite, sulfur dioxide (sulfur dioxide), iron sulfate. In practice, sodium hyposulfite (thiosulfate) is mainly used - Na2S203 5H20. Its amount is calculated depending on the amount of excess chlorine, based on the following reaction:
Na2S203 + C12 + H20 = Na2S04 + 2HCI + si.
According to the above binding reaction between active chlorine and sodium hyposulfite at a molar ratio of 1:1, 0.0035 g of sodium hyposulfite crystalline hydrate is used per 0.001 g of chlorine, or 3.5MrNa2S203-5H20 is used per 1 mg of chlorine.
Chlorination with preammonization. The chlorination method in preammonization is used:
1) in order to prevent the appearance of unpleasant specific odors that occur after chlorination of water containing phenol, benzene and ethylbenzene;
2) to prevent the formation of carcinogenic substances (chloroform, etc.) during chlorination of drinking water containing humic acids, methane hydrocarbons;
3) to reduce the intensity of the smell and taste of chlorine, especially noticeable in the summer;
4) to save chlorine with high chlorine absorption of water and the absence of odors, tastes and high bacterial contamination.
If natural water contains phenols (for example, due to pollution of water bodies with wastewater from industrial enterprises) even in small quantities1, then when disinfected with chlorine-containing compounds that are hydrolyzed to form hypochlorous acid, free active chlorine immediately interacts with phenol, forming chlorophenols, which even in small quantities concentrations give the water a birdy taste and smell. At the same time, the associated active chlorine - chloramine, having a lower redox potential, does not interact with phenol to form chlorophenols, and therefore the organoleptic properties of water do not deteriorate during disinfection. Similarly, free active chlorine is able to interact with hydrocarbons of the methane series with the formation of trihalomethanes (chloroform, dibromochloromethane, dichlorobromomethane), which are carcinogens. Their formation can be prevented by disinfecting water with bound active chlorine.
During chlorination with pre-ammonization, a solution of ammonia2 or its salts is first added to the water to be disinfected, and chlorine is introduced after 1-2 minutes. As a result, chloramines (monochloramines NH2C1 and dichloramines NHC12) are formed in the water, which have a bactericidal effect. Chemical reactions for the formation of chloramines are given on p. 170.
The ratio of the resulting substances depends on pH, temperature and the amount of reacting compounds. The efficiency of chlorination with preammonization depends on the ratio of NH3 and C12, and doses of these reagents are used in proportions of 1:2, 1:4, 1:6, 1:8. For the water of each source of water supply, it is necessary to select the most effective ratio. The rate of disinfection of water with chloramines is lower than the rate of disinfection with free chlorine, therefore, the duration of disinfection of water in the case of chlorination with preammonization should be at least 2 hours.
*MAC of phenol in water 0.001 mg/l, limiting indicator - organoleptic (smell), hazard class 4.*
*For the introduction of ammonia into water, it is most convenient to use vacuum chlorinators.*
But less oxidative activity, since the redox potential of chloramines is much lower than that of chlorine.
In addition to pre-ammonization (the introduction of ammonia 1-2 minutes before the introduction of chlorine), sometimes post-ammonization is used, when ammonia is injected after chlorine directly into tanks with clean water. Due to this, chlorine is fixed longer than the increase in the duration of its action is achieved.
Combined methods of water chlorination. In addition to the considered methods of water chlorination, a number of combined ones have been proposed, when another chemical or physical disinfectant agent is used together with chlorine-containing compounds, which increases the disinfection effect. Chlorination can be combined with water treatment with silver salts (chlorine-silver method), potassium permanganate (chlorination with manganation), ozone or ultraviolet light, ultrasound, etc.
Chlorination with manganation (with the addition of KMn04 solution) is used if it is necessary to enhance the oxidizing and bactericidal action of chlorine, since potassium permanganate is a stronger oxidizing agent. The method should be used in the presence of odors and flavors in the water, which are caused by organic substances, algae. In this case, potassium permanganate is introduced before chlorination. KMp04 should be added before settling tanks at doses of 1-5 mg/l or before filters at a dose of 0.08 mg/l. Recovering to water-insoluble MnO2, it is completely retained in sedimentation tanks and filters.
The silver chloride method is used on ships of the river fleet (at KVU-2 and VHF-0.5 units). It provides enhanced disinfection of water and its preservation for a long period (up to 6 months) with the addition of silver ions in the amount of 0.05-0.1 mg/l.
In addition, the silver chloride method is used to disinfect water in swimming pools, where it is necessary to reduce the dose of chlorine as much as possible. This is possible because the bactericidal action is provided within the limits of the total effect of doses of chlorine and silver.
The bactericidal, virucidal and oxidative effects of chlorine can be enhanced by simultaneous exposure to ultrasound, ultraviolet radiation, direct electric current.
Water samples are taken after clean water tanks before being supplied to the water supply network. Monitoring the effectiveness of chlorination by residual active chlorine is carried out hourly, that is, 24 times a day. Chlorination is considered effective if the content of residual free chlorine is in the range of 0.3-0.5 mg/l after 30 minutes of contact, or the content of residual combined chlorine is 0.8-1.2 mg/l after 60 minutes of contact.
According to microbiological indicators of epidemic safety, water after RCV is examined twice a day, that is, once every 12 hours. In water after disinfection, the total microbial number and the BGKP index (coli index) are determined. Water disinfection is considered effective if the coli index does not exceed 3, and the total microbial number is not more than 100.
Negative effects of water chlorination on public health. As a result of the reaction of chlorine with humic compounds, waste products of aquatic organisms and some substances of industrial origin, dozens of new extremely dangerous haloform compounds are formed, including carcinogens, mutagens and highly toxic substances with MPCs at the level of hundredths and thousandths of a milligram per 1 liter. In table. 3 and 5 (see p. 66, 67, 101) some halogen-containing compounds are given, the features of their effect on the human body, and hygienic standards in drinking water. The indicators of this group are trihalomethanes: chloro- and bromoform, dibromochloromethane, bromodichloromethane. In disinfected drinking water and hot water supply, chloroform is most often detected in higher concentrations - a carcinogen of group 2B, according to the IARC classification.
Haloform compounds enter the body with water not only enterally. Some substances penetrate intact skin during contact with water, in particular when swimming in a pool. While taking a bath or shower, haloform compounds enter the air. A similar process occurs in the process of boiling water, laundry, cooking.
Taking into account the extreme danger to human health of haloform compounds, a set of measures has been developed to reduce their levels in water. It provides:
Protection of the water supply source from pollution by wastewater containing precursors of haloform compounds;
Decreased eutrification of surface water bodies;
Rejection of rechlorination (primary chlorination) or its replacement with ultraviolet irradiation or the addition of copper sulfate;
Optimization of coagulation to reduce the color of water, that is, the removal of humic substances (precursors of haloform compounds);
The use of disinfectants that have a lower ability to form haloform compounds, in particular chlorine dioxide, chloramines;
Use of chlorination with preammonization;
Aeration of water or use of granular activated carbon is the most effective way to remove haloform compounds from water.
The cardinal solution of the problem is the replacement of chlorination with ozonation and disinfection of water with UV rays.
Ozonation of water and its advantages over chlorination. Ozonation is one of the promising methods of water treatment for its disinfection and improvement of organoleptic properties. Today, almost 1000 waterworks in Europe, mainly in France, Germany and Switzerland, use ozonation in their water treatment process. Recently, ozonation has been widely introduced in the United States and Japan. In Ukraine, ozonation is used at the Dnieper water pipeline
Rice. 25. Technological scheme of the ozonator plant:
1 - air inlet; 2 - air filter; 3 - warning valve; 4 - five supply fans; 5 - air plunger; 6 - two cooled dryers; 7 - four adsorption dryers; 8 - activated alumina; 9 - cooling fan heaters; 10 - fifty ozone generators (shown 2); 11 - dry air; 12 - cooling water inlet; 13 - outlet of cooling water; 14 - ozonized air; 15 - three tanks for ozone diffusion; 16 - water level
Stations in Kyiv, in the CIS countries - at waterworks in Moscow (Russian Federation) and Minsk (Belarus).
Ozone (Os) is a pale purple gas with a specific odor and a strong oxidizing agent. Its molecule is very unstable, easily decomposes (dissociates) into an atom and an oxygen molecule. Under industrial conditions, the ozone-air mixture is obtained in an ozonizer using a "slow" electric discharge at a voltage of 8000-10,000 V.
The schematic diagram of the ozonator plant is shown in fig. 25. The compressor takes in air, cleans dust, cools, dries on adsorbers with silica gel or active aluminum oxide (which are regenerated by blowing hot air). Then the air passes through the ozonator, where ozone is formed, which is fed through the distribution system into the water of the contact tank. The dose of ozone required for disinfection for most types of water is 0.5-6.0 mg/l. Most often, for underground water sources, the dose of ozone is taken in the range of 0.75-1.0 mg / l, for surface waters - 1-3 mg / l. Sometimes high doses are needed to decolorize and improve the organoleptic properties of water. The duration of contact of ozone with water should be at least 4 min1. indirect indicator
*In accordance with GOST 2874-82, the duration of water disinfection with ozone was at least 12 minutes. The same duration is regulated by SanPiN 2.1.4.559-96 approved by the Ministry of Health of Russia "Drinking water. Hygienic requirements for water quality in centralized drinking water supply systems. Quality control". In accordance with SanPiN "Drinking water. Hygienic requirements for the quality of water for centralized domestic drinking water supply", approved by the Ministry of Health of Ukraine, the duration of ozone treatment should be at least 4 minutes.*
The effectiveness of ozonation is the presence of residual amounts of ozone at the level of 0.1-0.3 mg/l after the mixing chamber.
Ozone in water decomposes, forming atomic oxygen: 03 -> 02 + O. It is proved that the mechanism of ozone decomposition in water is complex. In this case, a number of intermediate reactions occur with the formation of free radicals (for example, HO *), which are also oxidizing agents. More The strong oxidizing and bactericidal effect of ozone compared to chlorine is explained by the fact that its oxidizing potential is greater than that of chlorine.
From a hygienic point of view, ozonation is one of the best methods of water disinfection. As a result of ozonation, a reliable disinfecting effect is achieved, organic impurities are destroyed, and the organoleptic properties of water not only do not deteriorate, as with chlorination or boiling, but also improve: color decreases, excess taste and smell disappear, water acquires a blue tint. Excess ozone quickly decomposes to form oxygen.
Water ozonation has the following specific advantages over chlorination:
1) ozone is one of the strongest oxidizing agents, its redox potential is higher than that of chlorine and even chlorine dioxide;
2) when ozonizing, nothing extraneous is introduced into the water and there are no noticeable changes in the mineral composition of water and pH;
3) an excess of ozone turns into oxygen after a few minutes, and therefore does not affect the body and does not impair the organoleptic properties of water;
4) ozone, interacting with compounds contained in water, does not cause unpleasant tastes and odors;
5) ozone bleaches and deodorizes water containing organic substances of natural and industrial origin, giving it a smell, taste and color;
6) compared to chlorine, ozone more effectively disinfects water from spore forms and viruses;
7) the ozonation process is less affected by variable factors (pH, temperature, etc.), which facilitates the technological operation of water treatment facilities, and monitoring the efficiency is not more difficult than with water chlorination;
8) water ozonation ensures uninterrupted water treatment process, there is no need to transport and store unsafe chlorine;
9) during ozonization, significantly less new toxic substances are formed than during chlorination. These are mainly aldehydes (for example, formaldehyde) and ketones, which are formed in relatively small quantities;
10) ozonation of water enables complex water treatment, in which disinfection and improvement of organoleptic properties (color, smell and taste) can be simultaneously achieved.
Disinfection of water with silver ions. Water treated with silver at a dose of 0.1 mg/l maintains high sanitary and hygienic indicators throughout the year. Silver can be introduced directly by providing contact of water with the surface of the metal itself, as well as by dissolving silver salts in water in an electrolytic way. L.A. Kulsky developed ionizers LK-27, LK-28, which provide for the anodic dissolution of silver by electric direct current.
The mechanism of action of chemical disinfectants on microorganisms. The initial stage of the action of any disinfectant on a bacterial cell is its sorption on the cell surface (O.S. Savluk, 1998). After diffusion of disinfectants through the cell wall, the targets of their action are the cytoplasmic membrane, nucleoid, cytoplasm, ribosomes, mesosomes. The next stage is the degradation of macromolecular, including protein, structures of a bacterial cell as a result of inactivation of highly reactive functional groups (sulfhydryl, amine, phenolic, indole, thioethyl, phosphate, keto groups, endocyclic nitrogen atoms, etc.). The most sensitive are enzymes containing SH-groups, i.e., thiol enzymes. Among them, dehydrogenases, which ensure the respiration of bacteria and are localized mainly in mesosomes, are most strongly inhibited.
Among the organelles of a bacterial cell, one of the most damaged by chemical disinfectants is the cytoplasmic membrane. This is due to its easy accessibility for an oxidizing agent (compared to other organelles) and the presence of a large number of active groups (including sulfhydryl groups), which are easily inactivated. Therefore, relatively small amounts of disinfectants are needed to damage the cytoplasmic membrane. Due to the importance of the functions of the cytoplasmic membrane for the life of a bacterial cell, its damage is extremely dangerous.
The nucleoid, the main part of which is a DNA molecule, despite the presence of reactive groups that are potentially able to interact with disinfectants, is inaccessible to their molecules and ions. This is due, firstly, to the difficulty of transporting the disinfectant from an aqueous solution to the nucleoid through the outer and cytoplasmic membranes of the bacterial cell, and hence to unproductive losses of disinfecting agents. Secondly, the presence of a primary hydration shell on the DNA surface becomes an obstacle for some disinfectants. In particular, this hydration shell is impermeable to cations.
A significant amount of disinfectant is necessary to inactivate ribosomes and polysomes that contain rRNA, due to their high concentration in the bacterial cell (compared to DNA).
Chemical disinfectants should have the widest possible range of bactericidal action and minimal toxicity to the body. Taking into account the mechanism of interaction with bacterial cells, chemical disinfectants are divided into two groups:
1. Substances that affect cellular structures due to chemical and physical effects, i.e. substances of a polar structure that contain lipophilic and hydrophilic groups (alcohols, phenols, cresols, detergents, polypeptide antibiotics). They dissolve fragments of cellular structures - membranes, violating their integrity and, accordingly, functions. Possessing a wide spectrum of bactericidal action due to the similarity of the structure of cell membranes in various prokaryotes, this class of disinfectants is effective only at high concentrations - from 1 to 10 M.
2. Substances that affect cellular structures due to chemical interaction. They can be divided into 2 subclasses: 1) substances that only inhibit the growth of bacteria; 2) substances that cause their death. The line between them is rather conditional and is largely determined by concentration. Disinfectants that cause cell death include almost all heavy metals that form hard-to-dissociate complexes with sulfhydryl groups, as well as cyan-anions that form hard-to-dissociate complexes with iron, thereby blocking the function of the terminal respiratory enzyme cytochrome oxidase. Disinfectants that inhibit the growth of bacteria, when interacting with functional groups of cellular compounds, either lead to their transformation (reversible under certain conditions) into other groups, or inhibit them due to the structural similarity of disinfectants with normal cellular metabolites.
The effectiveness of chemical disinfectants also depends on the possibilities of their transport through cellular structures to the target in the cell. In gracilic (gram-negative) and firmacute (gram-positive) bacteria, the shells have a different structure, with the main difference being that gracilic bacteria have an additional outer layer consisting of phospholipids, lipoproteins, and proteins. Both two- and three-layer structures of the shell provide high selectivity for the penetration of foreign substances into the cell from the outside.
In addition to transport restrictions, the effectiveness of chemical disinfectants can be affected by the electrolyte composition of the water to be disinfected. For example, when heavy metal cations are used for disinfection, the presence of some anions (C1~, Br", I", SO^~, POJ", etc.) and an alkaline environment can lead to the formation of poorly soluble, poorly dissociated compounds.
The interaction of disinfectants with cell metabolites and chemical compounds contained in it can also lead to a change in the physicochemical properties of the disinfectant. So, according to L.A. Kulsky (1988), the intracellular fluid contains almost 3 mg-eq/l of anions, up to 100 mg-eq/l HPOj "and almost 20 mg-eq/l SOj", which is quite enough to convert many disinfectants, for example, heavy cations metals, into slightly dissociated compounds.
The mechanism of bactericidal action makes it possible to explain the synergistic effects that are observed experimentally when disinfecting water with combinations of chemical disinfectants or through the physical influence and action of a chemical disinfectant. From the point of view of the considered mechanism, the action of one of the combination of disinfectants neutralizes the "sacrificial defense" system of the bacterial cell, after which the other disinfectant gains practically unimpeded access to the main targets and, interacting with them, inactivates the cell.
Thus, combinations of chemical disinfectants should have optimal bactericidal properties, in which one is able to irreversibly bind sulfhydryl groups of envelope proteins, and the other, which has highly selective transport properties, quickly diffuses into the cytoplasm of the cell and, interacting with DNA and RNA, inactivates the bacterial cell. Such highly effective combinations Disinfectants are systems C12: H202, C12: 03, C12: Ag+, I2: Ag+, etc. When the physical influence and action of a chemical disinfectant are combined, as a result of physical action on the bacterial cell membrane, its structure is disorganized or partially destroyed. This contributes to easier transportation of the chemical disinfectant to the cell targets and its further inactivation. The use of combinations of disinfectants is very effective in terms of inactivation of bacterial mutant cells, which are found in cell populations in the amount of 10-4-10-".
The considered mechanism of the bactericidal action of chemical disinfectants makes it possible to explain the patterns of inactivation of viruses and bacteriophages. In particular, the increased resistance to chemical disinfectants of bacteriophages compared to bacterial cells is explained by their presence in the bacterial cytoplasm and thus the low availability of most chemical disinfectants. Inactivation of viruses and bacteriophages by chemical disinfectants outside the bacterial cell is probably due to the denaturation of the protein coats of the virus and interaction with its enzyme systems located under the protein coats.
Disinfection of water by ultraviolet (UV) irradiation. Disinfection of water with UV rays refers to physical (reagentless) methods. Reagent-free methods have a number of advantages; their application does not change the composition and properties of water, unpleasant tastes and odors do not appear, and there is no need to transport and store reagents.
A bactericidal effect is exerted by a section of the UV part of the optical spectrum in the wavelength range from 200 to 295 nm. The maximum bactericidal action falls on 260 nm. Such rays penetrate through a 25 cm layer of clear and colorless water. Water is disinfected with UV rays fairly quickly. After 1-2 minutes of irradiation, vegetative forms of pathogenic microorganisms die. Turbidity and especially color, color and iron salts, reducing the permeability of water to bactericidal UV rays, slow down this process. That is, a prerequisite for reliable disinfection of water with UV rays is its preliminary clarification and discoloration.
Disinfect by UV irradiation using bactericidal lamps, mainly water from underground water sources, if the index is not more than 1000 CFU / l, the iron content is not more than 0.3 mg / l. Bactericidal installations are equipped on the suction and pressure lines of pumps of the II lift in
Rice. 26. Installation for water disinfection with UV rays (OB AKX-1):
A - cut; b - scheme of water movement through the chamber; 1 - viewing window; 2 - body; 3 - partitions;
4 - water supply; 5 - mercury-quartz lamp PRK-7; 6 - quartz cover in separate buildings or rooms. If the productivity of the waterworks is up to 30 m3 / h, installations with a non-submersible source of radiation in the form of low-pressure argon-mercury lamps are used. If the productivity of the station is 30-150 m3 / h, then installations with submersible high-pressure mercury-no-quartz lamps are used (Fig. 26).
When using low-pressure argon-mercury lamps, toxic by-products are not formed in water, while under the action of high-pressure mercury-quartz lamps, the chemical composition of water can change due to photochemical transformations of substances dissolved in water.
The disinfecting effect of bactericidal UV rays is due mainly to photochemical reactions, resulting in irreversible damage to the DNA of a bacterial cell. In addition to DNA, UV rays also damage other structural parts of the cell, in particular rRNA, cell membranes. The yield of bactericidal energy is 11% at the optimal length of most of the emitted waves.
Thus, bactericidal rays do not denature water and do not change its organoleptic properties, and also have a wider range of abiotic effects - they have a detrimental effect on spores, viruses and helminth eggs that are resistant to chlorine. At the same time, the use of this method of water disinfection complicates the operational control of efficiency, since the results of determining the microbial number and coli-index of water can be obtained only after 24 hours of incubation of crops, and the express method, which is similar to the determination of residual free or combined chlorine or residual ozone, does not exist in this case.
Disinfection of water by ultrasound. The bactericidal effect of ultrasound is mainly due to the mechanical destruction of bacteria in the ultrasonic field. Electron microscopy data indicate the destruction of the bacterial cell wall. The bactericidal effect of ultrasound does not depend on the turbidity (up to 50 mg/l) and the color of the water. It applies to both vegetative and spore forms of microorganisms and depends only on the intensity of vibrations.
Ultrasonic vibrations, which can be used for water disinfection, are obtained by piezoelectric or magnetostrictive methods. To obtain water that meets the requirements of GOST 2874-82 "Drinking water. Hygienic requirements and quality control", the ultrasound intensity should be about 2 W / cm2, the oscillation frequency should be 48 kHz per 1 s. Ultrasound with a frequency of 20-30 kHz destroys bacteria in 2-5 s.
Thermal disinfection of water. The method is used to disinfect a small amount of water in sanatoriums, hospitals, on ships, trains, etc. Complete disinfection of water and the death of pathogenic bacteria is achieved after 5-10 minutes of boiling water. For this type of disinfection, special types of boilers are used.
X-ray disinfection. The method involves the irradiation of water with short-wave X-ray radiation with a wavelength of 60-100 nm. Short-wave radiation penetrates deeply into bacterial cells, causing their significant changes and ionization. The method has not been studied enough.
Vacuum disinfection. The method involves the inactivation of bacteria and viruses under reduced pressure. The full bactericidal effect is achieved within 15-20 minutes. The optimal processing mode is at a temperature of 20-60 ° C and a pressure of 2.2-13.3 kPa.
Other physical methods of disinfection, such as treatment with y-irradiation, high-voltage discharges, low-power electric discharges, alternating electric current, are used to a limited extent due to their high energy intensity, equipment complexity, and also because of their insufficient knowledge and lack of information about the possibility of formation harmful side compounds. Most of them are currently at the stage of scientific development.
Disinfection of water in the field. The water supply system in the field must guarantee the receipt of high-quality drinking water that does not contain pathogens of infectious diseases. Of the technical means suitable for improving water quality in the field, carbon-fabric filters (TUF) deserve special attention: they are portable, transportable, simple and highly productive.
Tuff designed by M.N. Klyukanov are intended for temporary use (water supply in the field, rural areas,
new buildings, during expeditions). Water is purified and disinfected according to the method of M.N. Klyukanov by simultaneous coagulation and disinfection with increased doses of chlorine (superchlorination) with further filtration through TUV (Fig. 27). Suspended particles are retained on the fabric filter layer, that is, water is clarified and discolored, and dechlorination is carried out on the carbon filter layer.
For coagulation, aluminum sulfate is used - A12 (S04) 3 in the amount of 100-200 mg / l. The dose of active chlorine for water disinfection (superchlorination) is at least 50 mg/l. At the same time, a coagulant and bleach or DTSGK (two-thirds basic salt of hypo-
Calcium chlorite) at doses of 150 and 50 mg/l, respectively. In this case, coagulation is not affected by the alkalinity of the water:
A) with bleach -
A12(S04)3 + 6CaOC12 + 6H20 -> -> 2A1(OH)3 + 3CaS04 + 3CaC12 + 6HOCI;
B) with DTSGK -
A12(S04)3 + 3Ca(OC1)2 2Ca(OH)2 + 2H20 -> 2A1(OH)3 + 3CaS04 + 2Ca(OC1)2 + 2HOC1.
Usually, coagulation occurs by the reaction of aluminum sulfate with water bicarbonates, which should be at least 2 mg-eq / l. In other cases, the water must be alkalized.
After 15 min after treatment with the above reagents, the settled water is filtered through TUF. Residual chlorine and organoleptic properties are determined in purified water.
Water supply network and facilities on it. The water supply network (water distribution system) is an underground pipe system through which water under pressure (at least 2.5-4 atm for a five-story building) created by a pumping station of the second lift is supplied to the settlement and bred on its territory. It consists of main conduits, through which water from the waterworks enters the settlement, and an extensive network of pipes, through which water is supplied to water tanks, external water intake structures (street columns, fire hydrants), residential and public buildings. At the same time, the main conduit branches into several main ones, which, in turn, branch into street, yard and house. The latter are connected to the internal water pipe system of residential and public buildings.
Rice. 28. Scheme of the water supply network: A - dead-end scheme; B - ring diagram; a - pumping station; b - plumbing; c - water tower; d - populated quarters; d - distributing network
According to the configuration, the water supply network can be: 1) ring; 2) dead end; 3) mixed (Fig. 28). The dead-end network consists of separate deaf lines into which water enters from one side. If such a network is damaged in any area, the water supply to all consumers that are connected to the line located behind the point of damage in the direction of water movement is interrupted. At the dead ends of the distribution network, water may stagnate, sediment may appear, which serves as a favorable environment for the reproduction of microorganisms. A dead-end water supply network, as an exception, is equipped on small township and rural water supply systems.
The best from a hygienic point of view is a closed water supply network, which consists of a system of adjacent closed circuits, or rings. Damage in any area does not lead to a cessation of the water supply, as it can flow through other lines.
The distribution system of the water supply system must ensure an uninterrupted supply of water to all points of its consumption and prevent water pollution along the entire path of its supply from the main water supply facilities to consumers. To do this, the water supply network must be waterproof. Pollution of water in the water supply network during centralized water supply is caused by: a violation of the tightness of water pipes, a significant decrease in pressure in the water supply network, which leads to suction of pollution in leaky areas, and the presence of a source of pollution near the area of leakage of water pipes. It is unacceptable to combine drinking water supply networks with networks supplying non-potable water (technical water supply).
Water pipes are made of cast iron, steel, reinforced concrete, plastics, etc. Pipes made of polymeric materials, as well as internal anti-corrosion coatings, are used only after their hygienic assessment and obtaining permission from the Ministry of Health. Steel pipes are used in areas with an internal pressure of more than 1.5 MPa, at the intersection with railways, roads, surface water bodies (rivers), at the intersection of the drinking water supply with sewerage. They need to protect the outer and inner surfaces from corrosion. The diameter of the drinking water pipes in urban areas must be at least 100 mm, in rural areas - more than 75 mm. A tight connection of individual pipe sections 5-10 m long is achieved using flanges, sockets or couplings (Fig. 29). Flanged connections are used only for open (on the surface of the earth) laying of pipes, where they are available for external inspection and leak testing.
The laying of water lines for household and drinking water supply should be preceded by a sanitary assessment of the territory by at least 40 m in both directions when the water supply is located in an undeveloped area and 10-15 m in a built-up area. The soil on which the water supply route will be laid must be uncontaminated. The route should not be laid through swamps, landfills, cemeteries, cattle burial grounds, that is, where the soil is contaminated. Along the water pipes, it is necessary to organize a sanitary protection zone (see pp. 129, 130).
Water pipes should be laid 0.5 m below the level of zero temperature distribution in the soil (soil freezing level). At the same time, depending on the climatic region, the depth of laying pipes varies from 3.5 to 1.5 m. In the southern regions, in order to prevent overheating of water in the summer, the depth of laying water pipes should be such that the soil layer above the pipe is at least 0 thick, 5 m
Water lines should be laid 0.5 m above sewer lines. If water pipes are laid on the same level as sewer lines laid in parallel, the distance between them must be at least 1.5 m for water pipes with a diameter of up to 200 mm and at least 3 m for water pipes with a diameter of more than 200 mm. In this case, metal pipes must be used. Metal water pipes are also used at their intersection with sewer lines. In this case, water pipes should be laid 0.5 m above sewer pipes. As an exception, at intersections, water pipes can be located below sewer pipes. At the same time, only steel water pipes are allowed to be used, additionally protecting them with a special metal casing with a length of at least 5 m on both sides of the intersection in clay soils and at least 10 m in soils with high filtration capacity (for example, sandy). Sewer pipes in the specified area must be cast iron.
On conduits and lines of the water supply network, the following are installed: butterfly valves (bolts) to highlight repair areas; plungers - for air release during operation of pipelines; valves - for the release and admission of air when the pipelines are freed from water for the period of repair and subsequent filling; releases - for dropping water when emptying pipelines; pressure regulators, valves for protection against hydraulic shocks, if it is suddenly necessary to turn off or turn on pumps, etc. The length of repair sections when laying water pipes in one line should not exceed 3 km, in two lines or more - 5 km.
Shut-off, control and security fittings are installed in inspection water wells. Inspection wells are also equipped at all junctions of the main, main and street water pipes. Wells are watertight reinforced concrete mines located underground. For descent into the manhole, a hatch with a hermetically sealed lid is provided, which is insulated during the cold season; cast-iron or steel brackets are mounted in the wall. The danger of contamination of water in the water supply network through manholes arises when the mine is filled with water. This can occur as a result of water entering through leaky walls and bottom, storm water through a leaky closed lid, or water from the water supply network through leaky pipe and fitting joints. During the decrease in pressure in the network, the water that has collected in the manhole can be sucked into the pipes.
Water pressure (spare) tanks are designed to create a supply of water that compensates for the possible discrepancy between the supply of water and its consumption at certain hours of the day. The tanks are filled mainly at night, and during the day during hours of intensive water use, water from them enters the network, normalizing the pressure.
Water tanks are installed at the highest point of the relief on towers towering above the tallest buildings of the settlement (Fig. 30). The area around the water towers is fenced off. Tanks must be watertight, made of iron or reinforced concrete. For cleaning, repairing and disinfecting the inner surface of the tank
Rice. 30. Water tower: a - appearance; b - section: I - supply and distribution pipe; 2 - overflow pipe
Hatches with tightly closed and sealed covers are provided. For air exchange of tanks, ventilation openings are equipped, closed with nets and protected from atmospheric precipitation. On the pipes supplying and discharging water, taps are installed for sampling water in order to control its quality before and after the tank. Water tanks need periodic (1-2 times a year) disinfection.
On large water pipelines, spare tanks - clean water tanks - are equipped underground. Of these, water is supplied to the water supply network by pumping stations of the III lift.
Water columns. The population takes water from the water distribution system or through house inlets and taps of the intra-house water supply network, or through external water-folding structures - columns.
Street water columns are the most vulnerable elements of the water supply system. There are many cases of epidemic outbreaks of infectious diseases, which are called the "one-column" epidemic.
There are different column designs, but the most common are Cherkunov and Moscow type systems. They are installed in building areas without introducing pipes of a centralized domestic and drinking water supply into structures. At the same time, the service radius of the column should be no more than 100 m. Recently, in cities with centralized water supply with water intake from surface water bodies, columns are widely used to organize pump room artesian water supply1.
The water column of the Cherkunov system (Fig. 31) consists of ground and underground parts. The underground part (manhole) looks like a shaft with waterproof reinforced concrete walls and bottom. An ejector is placed there (it is installed on the path of water movement from the water main to the inner water tube of the column) and a drain tank with an air tube. A hermetically sealed hatch is located in the reinforced concrete floor of the mine. The ground part of the column has an outlet pipe and a handle, which is connected by a rod to a valve located in front of the ejector at the outlet of water from the water main. Around the column, within a radius of 1.5-2 m, a blind area is equipped with an inclination from the column, under the outlet pipe - a tray for draining water spilled during use.
When the handle is pressed, the valve opens, and the water from the water main under pressure rises through the water pipe and pours out through the outlet pipe of the column. When the handle is released, the valve closes. Since the water remaining in the water pipe freezes during the cold season and breaks the pipe, it is provided for draining into a metal tank at the bottom of the manhole. In this case, the air from the tank through the air tube enters the mine. When the handle is pressed again and the valve is opened, the water, exiting under pressure through the narrowed opening of the water main into the water pipe, activates the ejector. The effect of ejection (suction), which occurs in the first seconds after the valve is opened and does not last long, sucks water from the tank into the water pipe. The tank through the air pipe is filled with air from the mine. Thus, the first portions of water coming from the column immediately after pressing the handle are a mixture of water from the water supply network and the drain tank. Due to the suction of water from the tank, the pressure in the ejector equalizes, the ejection effect disappears, after which water is supplied to the consumer exclusively from the water supply network. When the handle is released, the tank refills with water from the column water tube.
A real threat of contamination of the water in the column can arise if the well of the column is filled with water. The ways in which water enters the mine can be different. Thus, precipitation and surface runoff
* Pump-room water supply is carried out at the expense of local water supply. Its elements are: 1) underground interlayer (preferably artesian) source of class I according to GOST 2761-84; 2) artesian well; 3) underground pumping station with a submersible centrifugal pump; 4) penstock; 5) a pump room with standpipes (mainly of the Moscow type). Pump-room artesian water supply is widespread in Kyiv, where centralized water supply is provided by the Dnieper and Desnyansky river and artesian water pipelines.*
Rice. 31. Water column of the Cherkunov system: 1 - detail of the ejector and tank; 2 - injector; 3 - clutch; 4 - narrowed end of the water pipe; 5 - counterweight; 6 - tray; 7 - plaster; 8 - flooring from boards; 9 - air tube; 10 - water pipe; 11 - ejector; 12 - staples; 13 - rod; 14 - sand; 15 - valve (38 mm); 16 - stopcock; 17 - tank
They can penetrate into the manhole through a loose ceiling or leaky hatch. In case of violation of the integrity of the reinforced concrete walls and the bottom of the mine, water can come from the soil (soil moisture, which is formed during the filtration of atmospheric and melt water), especially at a high level of standing groundwater. The mine can be flooded with water from the water supply network. This happens when the pressure in the network drops below 1 atm. Wherein
Transparency and increased color worsen the organoleptic properties of well and spring water, limit its use, and sometimes indicate water pollution as a result of errors in the equipment of water intake facilities (wells or spring cappings), their improper placement relative to potential sources of pollution, or improper operation. Sometimes the cause of a decrease in transparency and an increase in the color of well and spring water can be a high concentration of iron salts (over 1 mg / l).
In well water, which is epidemically safe, the BGKP index usually does not exceed 10 (if the titer is not less than 100), the microbial number is not more than 400 in 1 cm3. With such sanitary and microbiological indicators in water, pathogens of intestinal infections with a water transmission factor are not determined.
The content of nitrates in well and spring water should not exceed 45 mg / l, in terms of nitrate nitrogen - 10 mg / l. Exceeding this concentration can cause water-nitrate methemoglobinemia (acute toxic cyanosis) in formula-fed infants due to the use of water with a high nitrate content for the preparation of nutrient mixtures. A slight increase in the level of methemoglobin in the blood without threatening signs of hypoxia can also be observed in children aged 1 to 6 years, as well as in the elderly.
An increase in the content of ammonium salts, nitrites and nitrates in well and spring water may indicate contamination of the soil through which the source water is filtered, as well as the fact that pathogenic microorganisms could enter simultaneously with these substances. With fresh pollution in the water, the content of ammonium salts increases. The presence of nitrates in water in the absence of ammonia and nitrites indicates a relatively long-term entry of nitrogen-containing substances into the water. With systematic pollution in water, both ammonium salts and nitrites and nitrates are detected. The intensive use of nitrogen fertilizers in agriculture also leads to an increase in the content of nitrates in groundwater. An increase in the permanganate oxidizability of groundwater above 4 mg/l indicates a possible contamination with easily oxidizable substances of mineral and organic origin.
Chlorides are one of the indicators of pollution of local water sources. At the same time, high concentrations (over 30–50 mg/l) of chlorides in water can be caused by their leaching from solonchak soils. Under such conditions, 1 liter of water can contain hundreds and thousands of milligrams of chlorides. Water with a chloride content of more than 350 mg/l has a salty taste and adversely affects the body. For a correct assessment of the origin of chlorides, one should take into account their presence in the water of neighboring similar water sources, as well as other indicators of pollution.
In some cases, each of these indicators may have a different nature. For example, the organic matter may be of plant origin. Therefore, water from a local source can be considered contaminated only under the following conditions: 1) not one, but several sanitary-chemical indicators of pollution are increased; 2) simultaneously increased sanitary and microbiological indicators of epidemic safety - microbial count and coli-index; 3) the possibility of contamination is confirmed by the data of the sanitary examination of the well or spring capturing.
Hygienic requirements for the placement and arrangement of mine wells. A shaft well is a structure with which the population collects groundwater and raises it to the surface. In the conditions of local water supply, it simultaneously performs the functions of a water intake, water-lifting and water-folding structures.
When choosing the location of the well, in addition to hydrogeological conditions, it is necessary to take into account the sanitary conditions of the area and the convenience of using the well. The distance from the well to the consumer should not exceed 100 m. The wells are placed along the slope of the area above all sources of pollution located both on the surface and in the soil. Under these conditions, the distance between the well and the source of pollution (ground filtration site, cesspool, compost, etc.) should be at least 30-50 m. If the potential source of pollution is located higher in the terrain than the well, then the distance between them is in the case of fine-grained soil, it should be at least 80-100 m, and sometimes even 120-150 m.
It is possible to scientifically substantiate the magnitude of the sanitary gap between a well and a potential source of soil pollution using the Saltykov-Belitsky formula, which takes into account local soil and hydrogeological conditions. The calculation is based on the fact that pollution, moving along with groundwater in the direction of the well, should not reach the place of water intake, that is, there should be enough time to disinfect the pollution. The calculation is made according to the formula:
Where L is the allowable distance between the source of pollution and the point of water intake (m), k is the filtration coefficient1 (m / day) is determined experimentally or according to tables, p, is the level of groundwater in the area of contaminated aquifer, is determined experimentally by a level; n2 - water level of the aquifer at the point of water intake; t is the required time for the movement of water between the pollution source and the water intake point (this time is assumed to be 200 days for bacterial pollution, and 400 days for chemical pollution); c is the active porosity of the soil2.
*Filtration coefficient - the distance that water travels in the soil, moving vertically down under the influence of gravity. Depends on the mechanical composition of the soil. It is 0.432 for medium-grained sands, 0.043 for fine-grained sands, and 0.0043 m / day for loams. *
*Active porosity is the ratio of the pore volume of a water-bearing rock sample to the total volume of the sample. Depends on the mechanical composition of the soil: for coarse-grained sands - 0.15, for fine-grained - 0.35. *
This formula is suitable for calculations only when the water-bearing rock is fine and medium-grained sands. If the water-bearing layer is represented by coarse-grained sands or even gravelly soils, the safety factor A should be added to the found value:
The coefficient is determined by the formula: A \u003d ai + a2 + a3, where a! - the radius of depression funnel1 is maximum for coarse-grained sands 300-400 m, for medium gravel - 500-600 m; а2 - distance to which the torch of pollution extends (depending on the power of the source of pollution, it ranges from 10 to 100 m); a3 - the value of the buffer zone that breaks the hydraulic connection between the plume of pollution and the peripheral end of the radius of the depression funnel (10-15 m).
A well is a vertical shaft of square or circular section (approximately 1 m2 in area) that reaches the aquifer (Fig. 33). The bottom is left open, and the side walls are fixed with a waterproof material (concrete, reinforced concrete, brick, wood, etc.). A layer of gravel 30 cm thick is poured onto the bottom of the well. The walls of the well must rise above the ground by at least 1 m. A clay castle and a blind area are equipped around the well to prevent seepage along the walls of the well (outside) pollution, which is washed out from the surface layers of the soil. To build a clay castle around the well, they dig a hole 2 m deep, 1 m wide and fill it with greasy clay. For a blind area around the ground part of the well, over the clay castle within a radius of 2 m, sand is added and poured with cement or concrete with a slope to divert atmospheric precipitation and water spilling from the well when using the well. To divert storm water, an intercepting ditch is arranged. Within a radius of 3-5 m around public wells, a fence should be made to restrict the access of vehicles.
It is desirable to carry out the rise of water from the well using a pump. If this is not possible, then they equip a rotator with a public bucket attached to it. It is unacceptable to use your own bucket, since this is associated with the greatest danger of water pollution in the well. The log cabin of the well is tightly closed with a lid and a canopy is made over the log cabin and the brace.
Captage is a special facility for collecting spring water (Fig. 34). The place of water outlet must be protected by waterproof walls and closed from above. To prevent surface runoff from entering the spring, diversion ditches are arranged. Around the walls of the captage, a lock of greasy clay and a blind area are equipped. Materials for capping structures can
* Depression funnel - a zone of low pressure that forms in a water-bearing rock when water is pumped out of a well due to the resistance exerted by the rock. Depends on the mechanical composition of the rock and the rate of pumping water.*
Rice. 33. General view of the shaft well: 1 - bottom three-layer filter; 2 - reinforced concrete rings made of porous concrete; 3 - reinforced concrete rings; 4 - cover; 5 - lase brackets; 6 - stone blind area; 7 - brace; 8 - clay castle; 9 - canopy cover
Be concrete, reinforced concrete, brick, stone, wood. So that the water in the captage does not rise above a certain level, an overflow pipe is equipped at this level.
Sanitation of mine wells. Sanitation of a mine well is a set of measures for the repair, cleaning and disinfection of a well in order to prevent water pollution in it.
For preventive purposes, the well is sanitized before putting it into operation, and then, if the epidemic situation is favorable, there is no pollution and complaints from the population about water quality, periodically once a year after cleaning and maintenance. It is mandatory to carry out
Rice. 34. Simple capturing of a descending spring: 1 - aquifer; 2 - waterproof layer; 3 - gravel filter; 4 - receiving chamber; 5 - viewing well; 6 - manhole manhole with a cover; 7 - ventilation hatch; 8 - partition; 9 - release into the sewer or ditch; 10 - pipe supplying water to the consumer
Preventive disinfection after a major overhaul of the well. Preventive sanitation consists of two stages: 1) cleaning and repair; 2) disinfection.
If there are epidemiological grounds to consider the well as a hotbed for the spread of acute gastrointestinal infectious diseases, and also if there is a suspicion (especially data) about water pollution with feces, animal corpses, and other foreign objects, sanitation is carried out according to epidemiological indications. Sanitation according to epidemiological indications is carried out in three stages: 1) preliminary disinfection; 2) cleaning and repair; 3) final disinfection.
The method of sanitation of mine wells. Sanitation according to epidemiological indications begins with disinfection of the underwater part of the well in a volumetric way. To do this, determine the volume of water in the well and calculate the required amount of bleach or calcium hypochlorite according to the formula:
Where P is the amount of bleach or calcium hypochlorite (g), E is the volume of water in the well (m3); C - the specified concentration of active chlorine in the well water (100-150 g / m3), sufficient to disinfect the walls of the log house and the gravel filter at the bottom, H - the content of active chlorine in bleach or calcium hypochlorite (%); 100 is a constant numerical factor. If the water in the well is very cold (+4 °С ... +6 °С), the amount of chlorine-containing preparation for disinfecting the well is doubled by volume. The calculated amount of the disinfectant is dissolved in a small volume of water in a bucket until a uniform mixture is obtained, clarified by settling and this solution is poured into the well. The water in the well is well mixed for 15-20 minutes with poles or by frequent lowering and raising the bucket on a cable. Then the well is closed with a lid and left for 1.5-2 hours.
After preliminary disinfection, water is completely pumped out of the well with a pump or buckets. Before a person descends into the well, they check if CO2 has accumulated there, for which a lighted candle is lowered in a bucket to the bottom of the well. If it goes out, then you can only work in a gas mask.
Then the bottom is cleaned from silt, dirt, debris and random objects. The walls of the log house are mechanically cleaned of dirt and fouling and, if necessary, repaired. Dirt and silt selected from the well are placed in a pit at a distance of at least 20 m from the well to a depth of 0.5 m, poured with 10% bleach solution or 5% calcium hypochlorite solution and buried.
For final disinfection, the outer and inner surfaces of the log house are irrigated from a hydro-panel with a 5% bleach solution or a 3% calcium hypochlorite solution at the rate of 0.5 dm3 per 1 m2 of area. Then they wait until the well is filled with water to the usual level, after which the underwater part of it is disinfected by volumetric method at the rate of 100-150 mg of active chlorine per 1 liter of water in the well for 6-8 hours. After the specified contact time, a water sample is taken from the well and check it for the presence of residual chlorine or do an odor test. If there is no smell of chlorine, add 1/4 or 1/3 of the initial amount of the drug and leave for another 3-4 hours. After that, a water sample is taken and sent to the laboratory of the territorial SES for bacteriological and physico-chemical analysis. At least 3 studies should be carried out, 24 hours each.
Disinfection of a well for preventive purposes begins with determining the volume of water in the well. Then they pump out water, clean and repair the well, disinfect the outer and inner parts of the log house by irrigation, wait until the well is filled with water, and disinfect the underwater part in a volumetric way.
Disinfection of water in the well with the help of dosing cartridges. Among the measures to improve the local water supply, an important place is occupied by the continuous disinfection of water in the well with the help of dosing cartridges. Indications for this are: 1) non-compliance of microbiological indicators of water quality in the well with sanitary requirements; 2) the presence of signs of water pollution in terms of sanitary and chemical indicators (they are disinfected until the source of pollution is identified and positive results are obtained after sanitation); 3) insufficient improvement in water quality after disinfection (sanation) of the well (if the titer is below 100, if the index is above 10); 4) in the foci of intestinal infections in the settlement after the disinfection of the well until the elimination of the focus. Disinfect the water in the well with the help of a dosing cartridge only by specialists of the territorial SES, while at the same time monitoring the quality of the water in terms of sanitary-chemical and microbiological indicators.
Dosing cartridges are cylindrical ceramic containers with a capacity of 250, 500 or 1000 cm3. They are made from: fireclay clay, diatomaceous earth (Fig. 35). Bleach or calcium hypochlorite is poured into cartridges and immersed in a well. Quantity
Rice. 35. Dosing cartridge
The chlorine-containing substances required for water disinfection depend on many factors. These include: the initial quality of groundwater, the nature, degree of pollution and the volume of water in the well, the intensity and mode of water withdrawal, the rate of groundwater inflow, the flow rate of the well. The amount of active chlorine also depends on the sanitary condition of the well: the amount of bottom silt, the degree of contamination of the log house, etc. It is known that the causative agents of intestinal infections in the bottom silt find favorable conditions and remain alive for a long time. That is why long-term disinfection (chlorination) of water using dosing cartridges cannot be effective without preliminary cleaning and disinfection of the well.
The amount of calcium hypochlorite with an activity of at least 52%, necessary for long-term disinfection of water in a well, is calculated by the formula:
X, \u003d 0.07 X2 + 0.08 X3 + 0.02 X4 + 0.14 X5,
Where X, - the amount of the drug required to load the cartridge (kg), X2 - the volume of water in the well (m3), is calculated as the product of the cross-sectional area of \u200b\u200bthe well and the height of the water column; X3 - well flow rate (m3/h), determined experimentally; X4 - water intake (m3 / day), established by polling the population; X5 - chlorine absorption of water (mg/l), determined experimentally.
The formula is given to calculate the amount of calcium hypochlorite containing 52% available chlorine. In the case of disinfection with bleach (25% active chlorine), the calculated amount of the drug should be doubled. When disinfecting water in a well in winter, the estimated amount of the drug is also doubled. If the content of active chlorine in the disinfectant is lower than the calculated value, then recalculation is carried out according to the formula:
Where P is the amount of bleach or calcium hypochlorite (kg); X! - the amount of calcium hypochlorite calculated according to the previous formula (kg); H, - the content of active chlorine in calcium hypochlorite, taken into account (52% o); H2 - the actual content of active chlorine in the preparation - calcium hypochlorite or bleach (%). In addition, when disinfecting water in a well in winter, the estimated amount of the drug is doubled. To determine the flow rate - the amount of water (in 1 m3), which can be obtained from the well in 1 hour, is quickly pumped out for a certain time
Water from it, measuring its amount, and recording the recovery time of the initial water level. The flow rate of the well is calculated by the formula:
Where D is the flow rate of the well (m3/h), V is the volume of pumped water (m3); t is the total time, consisting of the time of pumping out and restoring the water level in the well (min); 60 is a constant factor.
Before filling, the cartridge is preliminarily kept in water for 3-5 hours, then filled with a calculated amount of a disinfectant chlorine-containing preparation, 100-300 cm3 of water are added and thoroughly mixed (until a uniform mixture is formed). After that, the cartridge is closed with a ceramic or rubber stopper, suspended in a well and immersed in the water column approximately 0.5 m below the upper water level (0.2-0.5 m from the bottom of the well). Due to the porosity of the walls of the cartridge, active chlorine enters the water.
The concentration of active residual chlorine in the well water is monitored 6 hours after the dosing cartridge is immersed. If the concentration of active residual chlorine in the water is below 0.5 mg/l, it is necessary to immerse an additional cartridge and then carry out an appropriate control of the effectiveness of the disinfection. If the concentration of active residual chlorine in the water is significantly higher than 0.5 mg/l, remove one of the cartridges and carry out an appropriate control of the disinfection efficiency. In the future, the concentration of active residual chlorine is monitored at least once a week, also checking the microbiological indicators of water quality.