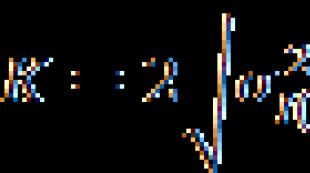Methodology for determining COD. Determination of COD and BOD of wastewater. Measurement procedure
CHEMICAL CONSUMPTION OF OXYGEN IN WATERS.
BY TITRIMETRIUM METHOD
Rostov-on-Don
2007
Preface
1 DEVELOPED BY SI “Hydrochemical Institute”
2 DEVELOPERS L.V. Boeva, Ph.D. chem. Sciences, T.S. Evdokimova
3 AGREED with UMZA and State Institution “NPO Typhoon” of Roshydromet
4 APPROVED by the Deputy Head of Roshydromet on March 13, 2007.
5 CERTIFIED by the State Hydrochemical Institute, certificate of certification No. 75.24-2006 dated October 2, 2006.
6 REGISTERED BY GU "NPO "Typhoon" under the number RD 52.24.421-2007
7 INSTEAD RD 52.24.421-95 “Methodological instructions. Methodology for measuring chemical oxygen consumption in waters.”
Introduction
Chemical oxygen demand (COD) is the amount of oxygen consumed for the oxidation of organic and inorganic substances contained in water by strong oxidizing agents. Depending on the nature of the oxidizing agent, permanganate, dichromate, iodate, and cerium oxidation are distinguished. If we eliminate the influence of inorganic substances or make corrections for their content, then the COD value characterizes the total concentration in water of organic substances oxidized under the analysis conditions by a given oxidizer. The highest degree of oxidation is achieved in a boiling acidic solution of potassium dichromate containing a catalyst. The amount of oxygen in milligrams per cubic decimeter, equivalent to the consumption of dichromate for the oxidation of organic substances, is called “dichromate oxidability”. Most often, when using the term “COD”, they mean the value of dichromate oxidability. Since the degree of oxidation of most organic substances with potassium dichromate under the indicated conditions is close to 100%, the value of dichromate oxidation correlates well with the mass concentration of organic carbon (the latter value is approximately 2.5 times less than COD).
COD is a generally accepted, important and fairly quickly determined indicator for characterizing the pollution of natural and waste waters with organic compounds. The COD (bichromate oxidability) values of surface waters, depending on the overall biological productivity of the water body, the degree of its pollution, as well as the content of organic substances of natural origin, range from fractions to tens of milligrams per cubic decimeter. Wastewater COD can reach hundreds of milligrams per cubic decimeter. There is a distinction between COD of filtered samples, indicating the content of dissolved organic substances, and unfiltered samples, indicating the total content of organic substances.
The oxidation of unpolluted surface waters of land exhibits a distinct physiographic zonation. Mountain regions are characterized by low oxidation - up to 5 mg/dm 3; average oxidation (from 5 to 10 mg/dm 3) occurs in zones of broad-leaved forests, forest-steppe, semi-desert, desert, tundra; increased (from 15 to 20 mg/dm 3) - in the northern and southern taiga zone.
The COD value is subject to quite significant and regular seasonal fluctuations. Their character is determined, on the one hand, by the hydrological regime and the dependent supply of organic substances of allochthonous origin from the surface of the catchment area, on the other hand, by hydrobiological activity, which determines the processes of production, transformation and mineralization of organic substances in a water body. In water bodies subject to strong anthropogenic impact, changes in the COD value are significantly influenced by the volume and mode of wastewater inflow.
GUIDANCE DOCUMENT
CHEMICAL CONSUMPTION OF OXYGEN IN WATERS.
MEASUREMENT PROCEDURE
BY TITRIMETRIUM METHOD
Date of introduction - 2007-04-01
1 area of use
1.1 This guidance document establishes a methodology for performing measurements (hereinafter referred to as the methodology) of chemical oxygen demand (COD) in samples of terrestrial surface waters and treated wastewater by the titrimetric method at organic content equivalent to oxygen consumption in the range from 4.0 to 80.0 mg/ dm 3. If the COD value is more than 50 mg/dm 3, measurements should be carried out with appropriate dilution of the sample with distilled water.
1.2 This guidance document is intended for use in laboratories analyzing terrestrial surface waters and treated wastewaters.
2 Normative references
This guidance document uses references to the following regulatory documents:
3 Assigned measurement error characteristics
3.1 Subject to all measurement conditions regulated by the methodology, the error characteristics of the measurement result with a probability of 0.95 should not exceed the values given in the table.
Table 1 - Measurement range, values of error characteristics and its components
When performing measurements in samples with a COD value over 80.0 mg/dm 3 after appropriate dilution, the measurement error does not exceed D × h, where D - error in measuring COD in a diluted sample; h - degree of dilution.
The detection limit of COD by the titrimetric method is 3 mg/dm 3 .
3.2 Method accuracy indicator values are used when:
Registration of measurement results issued by the laboratory;
Assessing the activities of laboratories for the quality of measurements;
Assessing the possibility of using measurement results when implementing the technique in a specific laboratory.
4 Measuring instruments, auxiliary devices, reagents, materials
When performing measurements, the following measuring instruments and other technical means are used:
4.1.1 High-quality laboratory balances (II) accuracy class according to GOST 24104-2001.
4.1.2 Laboratory balances, medium (III) accuracy class according to GOST 24104-2001.
4.1.3 State standard sample of chemical oxygen consumption in water GSO 7425-97.
4.1.4 Volumetric flasks 2 accuracy classes 2, 2a according to GOST 1770-74
200 cm 3 - 1 pc.
500 cm 3 - 2 pcs.
4.1.5 Graduated pipettes, 2 accuracy classes 1, 2 according to GOST 29227-91, capacity: 1 cm 3 - 1 pc.
2 cm 3 - 1 pc.
4.1.6 Pipettes with one mark 2 accuracy classes 1, 2 according to GOST 29169-91 with capacity: 5 cm 3 - 1 pc.
10 cm 3 - 2 pcs.
20 cm 3 - 2 pcs.
25 cm 3 - 1 pc.
50 cm 3 - 2 pcs.
4.1.7 Burette 2 accuracy classes 1, 2 according to GOST 29251-91 with capacity:
25 cm 3 - 1 pc.
4.1.8 Measuring cylinders, versions 1, 3 according to GOST 1770-74, capacity:
25 cm 3 - 2 pcs.
50 cm 3 - 2 pcs.
100 cm 3 - 1 pc.
250 cm 3 - 1 pc.
1000 cm 3 - 1 pc.
4.1.9 Conical flasks versions 1, 2 according to GOST 25336-82 with capacity:
500 cm 3 - 5 pcs.
4.1.11 Chemical beakers, type B, version 1, THS according to GOST 25336-82 with a capacity of 100 cm 3 - 1 pc.
250 cm 3 - 1 pc.
400 cm 3 - 1 pc.
1000 cm 3 - 1 pc.
4.1.12 Laboratory funnels according to GOST 25336-82 with a diameter of 56 mm - 2 pcs.
4.1.13 Round-bottomed flasks K-1 or pear-shaped flasks Gr with a capacity of 250 cm 3 and reflux condensers with interchangeable cones (installations for determining COD) according to GOST 25336-82 - 10 pcs.
4.1.14 Weighing cups (bugs) SV-19/9, SV-24/10, SN-45/13 according to GOST 25336-82.
4.1.15 Dropper.
4.1.16 Desiccator version 2, body diameter 190 mm according to GOST 25336-82.
4.1.17 Glass rods.
4.1.15 Glass capillaries.
4.1.16 Flushing.
4.1.17 Sand bath.
4.1.18 Drying cabinet for general laboratory purposes.
4.1.19 Device for filtering samples using membrane or paper filters.
4.1.20 Light and dark glass containers for storing samples and solutions with a capacity of 0.1; 0.25; 0.5 and 1 dm 3.
4.1.21 Polyethylene containers for storing solutions with a capacity of 0.25 dm 3.
4.1.22 Household refrigerator.
It is allowed to use other types of measuring instruments and auxiliary devices, including imported ones, with characteristics no worse than those given in.
When performing measurements, the following reagents and materials are used:
4.2.1 Potassium hydrophthalate according to TU 6-09-4433-77, analytical grade. (in the absence of GSO).
4.2.2 Potassium dichromate (potassium dichromate) according to GOST 4220-75, chemical grade. or potassium dichromate, standard titer, with a molar concentration of the amount of substance equivalent (AQE) of 0.1 mol/dm 3 according to TU 6-09-2540-72.
4.2.3 Ferrous ammonium sulfate salt (Mohr's salt) (NH 4 ) 2 Fe(SO 4 ) 2 × 6H 2 O according to GOST 4208-72, analytical grade.
4.2.4 Silver sulfate according to TU 6-09-3703-74, analytical grade.
4.2.5 Mercury sulfate, analytical grade, or yellow mercury oxide, analytical grade.
4.2.6 Sodium hydroxide (sodium hydroxide) according to GOST 4328-77, analytical grade.
4.2.7 Dehydrated calcium chloride according to TU 6-09-4711-81, part.
4.2.8 Sulfuric acid according to GOST 4204-77, chemically pure.
4.2.9 N-phenylanthranilic acid according to TU 6-09-05-66-73, parts, or ferroin (C 12 H 8 N 2) 3 × FeSO 4 according to TU 6-09-05-1256-83, part d.a; or 1,10-phenanthroline, monohydrate C 12 H 8 N 2× H 2 O or sulfate C 12 H 8 N 2 × N 2 SO 4 according to TU 6-09-05-90-80, part.
4.2.10 Distilled water according to GOST 6709-72.
4.2.11 Membrane filters “Vladipor MFAS-OS-2”, 0.45 microns according to TU 6-55-221-1-29-89 or another type, equivalent in characteristics.
4.2.12 Ashless filters “blue tape” according to TU 6-09-1678-86.
It is allowed to use reagents manufactured according to other regulatory and technical documentation, including imported ones, with qualifications not lower than those specified in.
5 Measurement method
The measurements are based on the oxidation of organic substances with potassium dichromate in a solution of sulfuric acid when heated in the presence of a catalyst - silver sulfate. Excess potassium dichromate is titrated with a solution of Mohr's salt and, based on the titration results, the amount of potassium dichromate consumed for the oxidation of organic substances is found.
Most organic compounds are oxidized by 95 - 100% under analytical conditions. Aliphatic hydrocarbons with a straight carbon chain and some heterocyclic compounds are not completely oxidized. Highly volatile organic compounds can volatilize during boiling if their oxidation does not proceed quickly enough.
6 Safety and environmental requirements
6.1 When performing COD measurements in samples of terrestrial surface waters and treated wastewater, comply with the safety requirements established in national standards and relevant regulations.
6.2 According to the degree of impact on the body, harmful substances used when performing measurements belong to hazard classes 1, 2 and 3 according to GOST 12.1.007.
6.4 Harmful substances must be collected and disposed of in accordance with established rules.
7 Operator qualification requirements
Persons with secondary vocational education, who have worked in the laboratory for at least 6 months and have mastered the technique are allowed to perform measurements and process their results.
8 Measurement conditions
8.1 When performing measurements in the laboratory, the following conditions must be met:
Ambient air temperature (20 ± 5) °C;
Atmospheric pressure from 84.0 to 106.7 kPa (from 630 to 800 mm Hg);
Air humidity no more than 80% at 25 °C;
Mains voltage (220 ± 10) V;
AC power frequency (50 ± 1) Hz.
8.2 It is not recommended to work with organic solvents in the room where COD measurements are carried out.
9 Sampling and storage
Sampling is carried out in accordance with GOST 17.1.5.05 and GOST R 51592. Sampling equipment must comply with GOST 17.1.5.04 and GOST R 51592. Samples are placed in bottles with stoppers that do not contaminate the sample with organic compounds.
COD measurements, especially in contaminated waters, should be performed as soon as possible after sampling. If this is not possible, the samples are preserved by adding a solution of sulfuric acid (1:2) at the rate of 2 cm 3 for every 100 cm 3 of water sample and stored at a temperature not exceeding 5 ° C. For treated wastewater, the storage period is no more than a day, for contaminated surface water - no more than 3 days, for uncontaminated water - up to 5 days. The volume of the sample taken is at least 50 cm3.
Depending on the purpose of the analysis, COD measurements are performed on an unfiltered or filtered sample.
In the latter case, the sample is filtered immediately after sampling through a membrane filter, cleaned by boiling twice in distilled water, or a blue ribbon paper filter, washed with hot distilled water. The first portion of the filtrate is discarded.
10 Preparing to take measurements
10.1 Preparation of solutions and reagents
10.1.1 Potassium dichromate solution with a molar concentration of the amount of substance equivalent (AQE) of 0.25 mol/dm 3
Weigh, accurate to the fourth decimal place, 6.129 g of potassium dichromate, previously dried for 2 hours at 105 °C, transfer it quantitatively into a volumetric flask with a capacity of 500 cm 3, dissolve in distilled water and adjust the volume of the solution to the mark. The solution is stored in a tightly closed dark bottle for no more than 6 months.
10.1.2 Potassium dichromate solution with a molar concentration of KVE 0.025 mol/dm 3
Place 50 cm 3 of a solution of potassium dichromate with a molar concentration of KVE 0.25 mol/dm 3 in a volumetric flask with a capacity of 500 cm 3 and adjust the volume of the solution to the mark with distilled water.
If a titre standard is used to prepare a solution of potassium dichromate, the contents of the ampoule are transferred to a 500 cm 3 volumetric flask and dissolved in distilled water. Then, using a pipette with one mark, 25 cm 3 of the resulting solution is taken, placed in a volumetric flask with a capacity of 200 cm 3, adjusted to the mark with distilled water and mixed.
Store in a dark bottle with a ground-in stopper for no more than a month.
10.1.3 Mohr’s salt solution with a molar concentration of KVE 0.25 mol/dm 3
Transfer 49.0 g of Mohr's salt into a 500 cm 3 volumetric flask, dissolve it in distilled water, carefully add 10 cm 3 of concentrated sulfuric acid while stirring and, after cooling, adjust the volume of the solution to the mark with distilled water. Store in a tightly closed dark bottle for no more than 6 months.
10.1.4 Mohr’s salt solution with a molar concentration of KVE 0.025 mol/dm 3
Place 50 cm 3 of a solution of Mohr's salt with a molar concentration of ECE 0.25 mol/dm 3 in a volumetric flask with a capacity of 500 cm 3 and adjust the volume of the solution to the mark with distilled water. Store in a tightly closed dark bottle. The exact concentration of the solution is set daily or before a series of measurements in accordance with.
10.1.5 Indicator solution
N solution is used as an indicator-phenylanthranilic acid or ferroin (ferrous sulfate complex ( II ) with 1,10-phenanthroline).
To prepare a solution of N-phenylanthranilic acid, 0.25 g of the reagent is dissolved in 12 cm 3 of sodium hydroxide solution (to accelerate dissolution, it can be slightly heated) and diluted with distilled water to 250 cm 3.
To prepare a solution of finished ferroin, 2.43 g of indicator is dissolved in 100 cm 3 of distilled water. When preparing a solution of ferroin from 1,10-phenanthroline, 0.980 g of Mohr's salt (NH 4) 2 Fe(SO) is dissolved in 100 cm 3 of distilled water 4) 2× 6H 2 O, add 2.09 g of 1,10-phenanthroline monohydrate or 2.93 g of sulfate and stir until the latter dissolves.
The indicator solution is stored in a tightly closed dark glass bottle for no more than 3 months.
10.1.6 Sodium hydroxide solution
Dissolve 0.4 g of sodium hydroxide in 100 cm 3 of distilled water. The solution is stable when stored in tightly closed polyethylene containers.
10.1.7 Silver sulfate solution
Dissolve 5.0 g of silver sulfate in 1 dm 3 of concentrated sulfuric acid. The solution is stable.
Using a pipette with a capacity of 10 cm 3, take 10 cm 3 of a solution of potassium dichromate with a molar concentration of KVE 0.025 mol/dm 3, transfer it to a conical flask with a capacity of 500 cm 3, add 180 cm 3 of distilled water and 20 cm 3 of concentrated sulfuric acid. After cooling, add 3 - 4 drops of ferroin indicator or 10 drops of N-phenylanthranilic acid solution, and titrate with Mohr's salt solution with a molar concentration of KVE 0.025 mol/dm 3 until the color changes from bluish-green to red-brown when ferroin is used as an indicator and from red-violet to bluish-green when using N-phenylanthranilic acid. The titration is repeated and, if there is no discrepancy in titrant volumes of more than 0.05 cm 3, the average value is taken as the result. Otherwise, repeat the titration until results are obtained that differ by no more than 0.05 cm 3 .
The exact molar concentration of Mohr's salt solution is found using the formula
(1)
where M m is the molar concentration of Mohr's salt solution, mol/dm 3 KVE;
M d - molar concentration of potassium dichromate solution, mol/dm 3 KVE;
V d - volume of potassium dichromate solution taken for titration, cm 3;
V m - volume of Mohr's salt solution used for titration, cm3.
11 Taking measurements
11.1 Elimination of interfering influences
Chlorides, sulfides, and iron compounds interfere with the determination ( II ), nitrites and other inorganic substances that can be oxidized by dichromate in an acidic environment.
The interfering effect of chlorides at concentrations less than 300 mg/dm 3 is eliminated due to the presence of a catalyst (silver sulfate) in the sample. For high chloride contents, add mercury sulfate to the sample ( II ) at the rate of 100 mg for every 10 mg of chlorides.
Interfering influence of sulfides and iron compounds (II) are eliminated by pre-blowing the water sample with air if it does not contain volatile organic compounds, or are taken into account when calculating COD. In the latter case, their concentrations are determined and recalculated to COD values, based on the fact that 1.0 mg H 2 S and 1.0 mg Fe 2+ equivalent to 0.47 and 0.14 mg of oxygen, respectively. The effect of nitrites is taken into account in the same way - 1.0 mg NO - 2 (or 0.30 mg nitrite nitrogen) is equivalent to 0.35 mg oxygen.
If the chloride content in the analyzed water is less than 300 mg/dm 3, pipet 20 cm 3 of water (or an aliquot brought to 20 cm 3 with distilled water) into a round-bottomed or pear-shaped flask for boiling, add 10 cm 3 of a solution of potassium dichromate with a molar concentration of 0.025 mol /dm 3 KVE, 30 cm 3 solution of silver sulfate in concentrated sulfuric acid (or 150 mg of silver sulfate and 30 cm 3 concentrated sulfuric acid) and drop 2 - 3 capillaries for uniform boiling. If COD measurements are performed on an unfiltered sample, the sample is thoroughly mixed for 2 to 3 minutes before aliquoting.
A reflux condenser is connected to the flask and the mixture is boiled in a sand bath for 2 hours. After cooling, rinse the refrigerator with distilled water (about 50 cm 3), disconnect it, add another 50 cm 3 of distilled water to the flask, washing its walls, cool again, transfer the sample to a conical flask, rinsing the flask in which the sample was boiled twice with distilled water (20 - 30 cm 3 each).
Add 3 - 4 drops of ferroin solution (or 10 drops of phenylanthranilic acid solution) to the sample and titrate the excess of unreacted potassium dichromate with Mohr's salt solution until the color of the indicator changes from bluish-green to red-brown when ferroin is used as an indicator and from red-violet to bluish green when used N -phenylanthranilic acid.
A blank experiment is carried out in a similar way with 20 cm 3 of distilled water.
11.3 Performing measurements in waters with high chloride content
If the chloride content in water exceeds 300 mg/dm3, add mercury sulfate at the rate of 100 mg for every 10 mg of chlorides contained in the sample to the aliquot of the sample selected for analysis (20 cm3 or a smaller aliquot adjusted to 20 cm3) and mix thoroughly . Next, perform the determination as described in. The presence of a small amount of precipitate formed after the addition of mercury sulfate does not interfere with the determination. In the absence of mercury sulfate, you can use a suspension of mercury oxide in sulfuric acid at the rate of 70 mg for every 10 mg of chlorides. To prepare the suspension, measure 30 cm 3 of a solution of silver sulfate in concentrated sulfuric acid (or 30 cm 3 of concentrated sulfuric acid if silver sulfate is added separately) into a glass with a capacity of 100 cm 3 and add the calculated amount of mercury oxide. Stir the mixture with a glass rod, leave for 15 minutes, and then pour the resulting suspension into the sample.
12 Calculation and presentation of measurement results
12.1 COD value (dichromate oxidability) X , mg/dm 3, found by the formula
![]() (2)
(2)
where V 1 - volume of Mohr's salt solution used for titration of the blank experiment, cm 3 ;
V 2 - volume of Mohr's salt solution used for titration of the water sample, cm 3 ;
M is the molar concentration of Mohr's salt solution, mol/dm 3 KVE;
V is the volume of an aliquot of water sample taken for execution, cm 3;
8.0 is the mass of millimoles of oxygen ECE, mg/mmol.
12.2 The measurement result in documents providing for its use is presented in the form:
X ± D (P = 0.95), (3)
where ±D - limits of measurement error characteristics for a given COD value, mg/dm 3 (table).
12.3 It is acceptable to present the result in the form:
X ± D l (P = 0.95) provided D l< D , (4)
where ± D l - limits of the error characteristics of the analysis results, established during the implementation of the methodology in the laboratory and ensured by monitoring the stability of the measurement results, mg/dm 3.
Note - It is permissible to establish the characteristic of the error of measurement results when introducing a technique in a laboratory on the basis of the expression D l = 0.84 × D with subsequent clarification as information is accumulated in the process of monitoring the stability of measurement results.
The numerical values of the measurement result must end with a digit of the same digit as the values of the error characteristic; the latter must not contain more than two significant figures.
12.4 The measurement results are documented in a protocol or journal entry, according to the forms given in the Laboratory Quality Manual.
13 Quality control of measurement results when implementing the technique in the laboratory
13.1 General provisions
13.1.1 Quality control of method measurement results in the laboratory includes:
- (based on monitoring the stability of standard deviation of repeatability, error, intra-laboratory precision).
13.1.2 The frequency of monitoring by the contractor of the measurement procedure, as well as the implemented procedures for monitoring the stability of the results of measurements, are regulated in the Laboratory Quality Manual.
13.2 Algorithm for operational control of the measurement procedure using the additive method together with the sample dilution method
13.2.1 When carrying out operational control, GSO 7425-97 based on potassium hydrophthalate with a bichromate oxidability value corresponding to 10.0 mg/cm 3 of oxygen is used to introduce additives. In the absence of GSO, it is permissible to use a certified solution of potassium hydrophthalate (see Appendix).
13.2.2 Operational control by the performer of the measurement procedure is carried out by comparing the results of a separate control procedure K to with the control standard K.
13.2.3 The result of the control procedure K k, mg/dm 3, is calculated using the formula
K k = X" + (h - 1) × X ¢ - X - C d, (5)
where X " - h times, with a known additive, mg/dm 3 ;
X¢ - the result of a control measurement of the COD value in a sample diluted in h times, mg/dm 3 ;
X is the result of a control measurement of the COD value in the working sample, mg/dm 3 ;
C d - additive value, mg/dm3.
13.2.3 Control standard K, mg/dm3, is calculated using the formula
(6)
where D lx ² (D lx ¢ and D lx ) - values of the error characteristics of the measurement results, established during the implementation of the method in the laboratory, corresponding to the COD value in a diluted sample with an additive (diluted sample, working sample), mg/dm 3 .
Note - To calculate the control standard, it is permissible to use the values of the error characteristics obtained by calculation using the formulas D lx ¢ = 0.84 × D x ¢ and D lx = 0.84 × D x.
13.2.4 If the result of the control procedure satisfies the condition:
A.3.1 High-quality laboratory scales (II) accuracy class according to GOST 24104-2001.
A.3.2 Measuring flask 2 accuracy classes 2, 2a according to GOST 1770-74 with a capacity of 100 cm 3.
A.3.3 Weighing cup (jug) SV 19/9 according to GOST 25336-82.
A.3.4 Laboratory funnel according to GOST 25336-82 with a diameter of 56 mm.
A.3.5 Spatula.
A.3.6 Flushing.
A.3.7 Desiccator version 2 with a body diameter of 140 mm or 190 mm in accordance with GOST 25336-82 with anhydrous calcium chloride.
A.3.8 Drying cabinet for general laboratory purposes.
A.4 Initial components of the certified solution
A.4.1 Potassium hydrophthalate according to TU 6-09-4433-77, analytical grade, with a content of the main substance from 99.8 to 100.2%.
A.4.2 Distilled water according to GOST 6709-72.
A.5 Procedure for preparing a certified AR-COD solution
To prepare a certified solution, weigh in a weighing bottle accurate to the fourth decimal place 0.851 g of potassium hydrophthalate, previously dried in an oven at 110 °C for 2 hours and cooled in a desiccator to room temperature. Transfer the sample quantitatively into a 100 cm 3 volumetric flask, dissolve it in distilled water, adjust the volume of the solution in the flask to the mark and mix. Transfer the solution to a dark bottle with a well-ground glass stopper.
The resulting solution is assigned a COD value of 10.0 mg/cm 3 .
A.6 Calculation of metrological characteristics of the certified AR-CPC solution
The certified value of COD C, mg/cm 3, is calculated using the formula
![]() (A.1)
(A.1)
where m - mass of potassium hydrophthalate sample, g;
7.5 is the number of moles of oxygen required to oxidize one mole of potassium hydrophthalate;
32.0 and 204.2 - molar mass of oxygen and potassium hydrophthalate, respectively, g/mol;
Calculation of the error in preparing a certified AR-CPC solution D , mg/dm 3, perform according to the formula
 (A.2)
(A.2)
where C is the COD value assigned to the solution, mg/dm 3 ;
Dm - the limiting value of the possible deviation of the mass fraction of the main substance in the reagent from the assigned value m, %;
m - mass fraction of the main substance (potassium hydrophthalate) in the reagent, assigned to the analytical grade reagent, %;
Dm - maximum possible weighing error equal to 0.0002 g;
m is the mass of a sample of potassium hydrophthalate, g;
D V - the limit value of the possible deviation of the capacity of the volumetric flask from the nominal value, cm 3;
V is the capacity of the volumetric flask, cm 3.
The value of the limit of possible error values for the preparation of a certified solution is equal to
A.7 Safety requirements
General safety requirements when working in chemical laboratories must be observed.
A.8 Operator qualification requirements
A certified solution can be prepared by an engineer or laboratory technician with secondary specialized education, who has undergone special training and has worked in a chemical laboratory for at least 6 months.
A.9 Labeling requirements
A label indicating the symbol of the solution, the COD value, the error in its determination and the date of preparation must be affixed to the bottle with the certified solution.
A.10 Storage conditions
The certified solution should be stored in a dark glass bottle with a ground stopper in the refrigerator for no more than 3 months.
Federal Service for Hydrometeorology and Monitoring
environment
GOVERNMENT INSTITUTION
"HYDROCHEMICAL INSTITUTE"
CERTIFICATE No. 75.24-2006
about MVI certification
Measurement procedure chemical oxygen consumption in waters by titrimetric method,
developed GU "Hydrochemical Institute" (GU GHI)
and regulated RD 52.24.421-2007
certified in accordance with GOST R 8.563-96 as amended in 2002.
Certification was carried out based on the results experimental research
As a result of the certification, it was established that the method complies with the metrological requirements imposed on it and has the following basic metrological characteristics:
1 Range of measurements, values of the accuracy indicator and its components at a confidence level of P = 0.95
2. Measurement range, values of repeatability and reproducibility limits at confidence level P = 0.95
3 When implementing the technique in the laboratory, provide:
Control by the performer of the measurement procedure (based on the assessment of the error when implementing a separate control procedure);
Monitoring the stability of analysis results (based on monitoring the stability of standard deviation of repeatability, standard deviation of intra-laboratory precision, error).
The algorithm for monitoring the measurement procedure by the contractor is given in RD 52.24.421-2007.
The frequency of monitoring by the contractor of the measurement procedure, as well as the implemented procedures for monitoring the stability of the results of measurements performed, are regulated in the Laboratory Quality Manual.
Chief metrologist of the State Chemical Institute A.A. Nazarova
In the context of the topic of caring for the environment, the issue of keeping rivers and other bodies of water clean is often discussed. Now this is extremely difficult to do, because the wastewater that is discharged into water bodies is highly polluted.
After active participation in one or another industrial process, wastewater accumulates a huge amount of harmful elements, which, when released into an open body of water, lead to the death of aquatic inhabitants and plants, as well as other unpleasant consequences.
To measure the degree of pollution of wastewater, several indicators are taken as a basis, one of which is COD. What is COD and how to reduce this indicator, we will tell you in this material.
Why do we need indicators of the degree of wastewater pollution?
The amount of wastewater pollution can be identified by a number of indicators, the most common among them are:
- COD or chemical oxygen demand;
- BOD is its biochemical consumption.
Measuring an indicator such as COD is necessary to analyze the quality of wastewater or liquid in a reservoir or to study the state of waters in general. COD is a quantitative indicator, it is one of the most informative and detailed.
The following substances act as wastewater pollutants:
- dissolved;
- weighted.
 The method for studying the state of a liquid taking into account COD is to determine the amount of oxygen that was spent on the oxidation of organic matter and minerals containing carbon. COD is also called unit of chemical oxidability of water, since organic substances are oxidized by oxygen. After all, it, in turn, is one of the most powerful oxidizing agents.
The method for studying the state of a liquid taking into account COD is to determine the amount of oxygen that was spent on the oxidation of organic matter and minerals containing carbon. COD is also called unit of chemical oxidability of water, since organic substances are oxidized by oxygen. After all, it, in turn, is one of the most powerful oxidizing agents.
Oxidability, depending on the origin of the oxidizing agents, can be of the following types:
- iodate;
- bichromate;
- cerium;
- permanganate.
The most accurate indicators are determined by application of the dichromate or iodate method. Oxidability is expressed as the ratio of the volume of oxygen that was spent on the oxidation of mineral and organic substances. It is expressed in milligrams per 1 square meter. dm. liquids.
It is necessary to purify wastewater in order to reduce the concentration of harmful substances to normal levels, which are approved in regulatory documents.
Cleaning is carried out at special treatment facilities or stations. Their layout depends on the quantity and quality of wastewater, as well as the level of its contamination. However, the wastewater treatment scheme will be the same and the main goal of the work is to reduce COD and BOD.
COD and BOD as criteria for water pollution
The COD value includes the total content of organic substances in the liquid in the volume of consumed bound oxygen for their oxidation. COD is a general indicator of industrial and natural water pollution.
But such an indicator as BOD determines the amount of dissolved oxygen that is spent on the oxidation of organic substances by bacteria in the required volume of liquid.
For identical samples, the COD value will be higher than the BOD value, since more substances are subject to chemical oxidation.
What factors influence COD
 There are a lot of factors that can affect the composition of harmful substances and the acidity of a liquid. One of the key factors is a set of biochemical processes occurring in the reservoir itself. As a result of these processes, substances react with each other and form new ones, which may differ in structure from the previous ones and have a different chemical composition.
There are a lot of factors that can affect the composition of harmful substances and the acidity of a liquid. One of the key factors is a set of biochemical processes occurring in the reservoir itself. As a result of these processes, substances react with each other and form new ones, which may differ in structure from the previous ones and have a different chemical composition.
These substances can enter the reservoir as follows:
- along with precipitation;
- together with domestic or industrial wastewater;
- with underground and surface wastewater.
Their structure and composition can be very different, in particular, which of them can be resistant to oxidizing agents. Depending on this factor, you need to choose the most effective oxidizing agent for certain substances.
In surface waters, organic matter can be suspended, dissolved, or colloidal. Oxidability is different for filtered and unfiltered samples. Natural waters are less susceptible to pollution by organic matter of natural origin.
Surface waters have a higher degree of oxidation compared to such types of water as:
- underground;
- ground and others.
For example, mountain rivers and lakes have oxidation in the region of 2–3 mg per cubic decimeter, rivers fed by swamps - 20 mg/cubic meter. dm and flat reservoirs - from 5 to 12, respectively.
A significant factor that affects oxidation is seasonal changes occurring in hydrobiological and hydrological regimes.
Also, the oxidation of a reservoir can change under the influence of human activity; depending on the sphere of human activity, pollution of one type or another enters the reservoir.
Requirements for the COD indicator according to the standard

According to the standard, COD indicators should fluctuate ranging from 15 to 30 mg/cu.m. dm. The degree of wastewater pollution according to COD indicators looks like this:
- very pure – up to 2 mg/cu. dm;
- relatively pure – 3 mg/cu. dm;
- average pollution – 4 mg/cu. dm;
- contaminated – 15 mg/cubic dm. and higher.
Stages of wastewater treatment and reduction of pollution levels
Wastewater treatment includes the following stages:
- primary cleaning – This is the removal of oil films, large pieces of dirt and numerical contaminants that are easily removed. This stage involves cleaning using a physical-mechanical method;
- secondary treatment. At this stage, suspended parts and pollutants, which are contained even in dissolved form, are separated. Some pollutants are organic in origin and must be removed through biological oxidation. This stage involves a biological method of wastewater treatment;
- tertiary treatment – This is the removal of all remaining small particles and contaminants, including metal salts. Purification is carried out by osmosis, electrodialysis, filtration through an adsorbent, etc.;
- fourth stage – At this stage, the sludge is dewatered, which reduces its volume and weight to a minimum.
The level of COD and BOD is gradually reduced to certain values at each stage, the amount of their reduction depends on the characteristics of the wastewater.
Wastewater is not always treated in all four stages. Very often, treatment plants discharge wastewater into the collector after the first stage of treatment, and this brings COD levels back to normal. In some countries, purification is carried out in only two stages, the third stage being used only as a last resort.
The difference between domestic wastewater and industrial wastewater
Wastewater can be of industrial or domestic origin; the nature of the contaminants in them is also different. So, as a rule, household wastewater is contaminated with such things as:
- garbage;
- organic residues;
- detergents.
But industrial drains are filled with industrial waste, if it is the food industry, then there Most of all there will be suspended substances and fats. COD and BOD values in industrial wastewater will be higher than in domestic wastewater.
Sometimes wastewater is combined, as a result of which organic matter from domestic wastewater becomes a breeding ground for activated sludge from bioremediation.
Ranges of criteria ratios for different waters
An analysis of such an indicator as COD is carried out to determine how much oxygen equivalent to dichromate is contained, which was used to oxidize all organic and inorganic substances in the sample.
As mentioned earlier, a value such as COD, which evaluates the reducing activity of chemicals, will be greater than BOD, the value of which depends solely on the amount of organic matter subject to biochemical decomposition. The relationship between these two indicators reflects the completeness of biochemical oxidation of substances, which are contained in wastewater. The greater the difference between these indicators, the greater the increase in biologically active masses. In particular, this ratio can be used to determine how suitable the wastewater is for biological treatment.
 If there are few substances susceptible to biochemical oxidation, then it is best to use physicochemical methods for research that can bring the ratio of indicators to the required figure.
If there are few substances susceptible to biochemical oxidation, then it is best to use physicochemical methods for research that can bring the ratio of indicators to the required figure.
Optimal range the ratio of BOD and COD is from 0.4 to 0.75 units. The optimal value for the ratio between the chemical and biological demand for oxygen is 0.7, with which the biological treatment process can proceed fully and fully.
Once the wastewater is separated by gravity, it removes mainly those substances that are difficult to oxidize. After this stage, the ratio of indicators increases.
Then follows biological treatment stage, as a result of which the ratio of indicators decreases by 0.2, since organic substances that undergo biochemical oxidation disappear in wastewater.
Also, in order to assess the presence of biologically degradable particles in waters, the inverse ratio of indicators can be used. For example, according to sanitary requirements, which imply that the COD for wastewater suitable for biotreatment, this indicator should not exceed the BOD value by more than one and a half times.
 If we talk about biological treatment facilities that purify mixtures of domestic and industrial wastewater, then, as a rule, they have a ratio of both parameters in the incoming liquid for treatment is somewhere in the region of 1.5 to 2.5. When wastewater is mixed with industrial waste, this figure increases to 3.5, and when water flows from some production facilities it can reach up to 8.
If we talk about biological treatment facilities that purify mixtures of domestic and industrial wastewater, then, as a rule, they have a ratio of both parameters in the incoming liquid for treatment is somewhere in the region of 1.5 to 2.5. When wastewater is mixed with industrial waste, this figure increases to 3.5, and when water flows from some production facilities it can reach up to 8.
As you can see, the COD value will allow you to analyze the state of the liquid in reservoirs and make it possible to find out how suitable it is for purification and to what extent. Detailed research into this and other values will make the environment around us much cleaner.
Introduction
BOD is a mandatory analysis, but its frequent determination in factory conditions is difficult for a number of reasons.
COD is understood as the amount of oxygen dissolved in water, expressed in mg O per 1 liter of water, necessary for the oxidation reactions of organic compounds present in wastewater.
It is believed that BOD constitutes about 70% of the mass of oxygen required for the complete oxidation of organic substances in a water sample to CO 2 and H 2 O. When oxidizing wastewater with potassium permanganate (permanganate), the oxygen consumption (BOD 5) barely reaches 25% of its requirement for complete oxidation of organic substances compared to the dichromate method for determining oxidability (COD). Therefore, COD gives a more accurate estimate of the amount of organic impurities in water, and the COD value is higher than BOD5. In numerical terms, COD is usually 20 - 30% greater than BOD, and in potato starch plant wastewater the COD is more than twice the BOD, which is explained by their chemical composition.
The most complete determination of oxidizable organic substances is achieved by the bichromate method (Yu. Lurie method). Its disadvantage is long-term oxidation (two-hour boiling) and high consumption of concentrated sulfuric acid.
The Bratislava Water Resources Research Institute (Czech Republic) has developed an accelerated dichromate method for determining COD, which is currently used in domestic sugar factories.
Purpose of analysis– assess the quality of wastewater based on the results of their analyzes for COD.
Principle of analysis method based on the oxidation of organic substances in wastewater with potassium dichromate.
Reagents:
0.25 n. K Cr O solution: 12.258 g of K Cr O dried at a temperature of 105 ºС, dissolved in 1 dm 3 of distilled water;
0.25 n. Mohr's salt solution: dissolve 98 g of Mohr's salt in distilled water, add 20 cm 3 of concentrated H SO and, after cooling, bring to 1 dm 3 with distilled water;
Silver sulfate – crystalline, analytical grade;
Phenylanthranilic acid: dissolve 0.25 g of phenylanthranilic acid in 12 cm 3 0.1 N. NaOH solution and bring to 250 cm 3 with distilled water.
Devices and materials:
Erlenmeyer flask with a capacity of 100 cm 3;
Pipettes;
50 cm 3 cylinder;
Glass balls.
Progress of determination
A 10 cm 3 sample or its corresponding part, brought to a volume of 10 cm 3 with distilled water, is pipetted into an Erlenmeyer flask with a capacity of 100 cm 3 .
Then approximately 0.1 g of Ag SO catalyst is added, and exactly 5 cm 3 of 0.25 N is pipetted. solution of K Cr O, and from the cylinder with continuous stirring - 15 cm 3 of concentrated H SO.
Place capillaries or glass beads into the solution to allow a gentle boil and leave it for one minute. Next, 20 cm 3 of distilled water is added and the mixture is cooled.
After cooling, add 3–4 drops of N-phenylanthranilic acid and the excess of unreacted potassium dichromate is titrated with 0.25 N. solution of Mohr's salt (FeSO (NH)SO ·6HO) until light green color.
Then a blind experiment is done: take 10 cm 3 of distilled water and perform an analysis similar to a working experiment.
Calculations:
COD calculation is carried out according to the formula
Where α - quantity 0.25 n. solution of Mohr's salt, used for blind experiment (10 cm 3 of distilled water), cm 3; O taken for titration, cm 3;
X – quantity 0.25 n. solution of Mohr's salt, used for titration 25 cm 3 0.25 N. solution, cm 3.
One of the most common methods for assessing the degree of contamination of wastewater is the COD indicator (chemical oxygen absorption - Lurie Yu. Yu. Analytical chemistry of industrial wastewater. - M.: Chemistry, 1984.)
In the USSR, the bichromate method for determining COD was adopted as an arbitration method. However, this method is time-consuming (about 6 hours) and requires a large consumption of sulfuric acid (165 ml for each analysis), so it is not very suitable for mass analyzes in factory laboratories and wastewater treatment plants.
There are simpler, accelerated versions of this method, which, however, give somewhat lower results compared to the arbitration method. In addition, the known accelerated methods are not unified and need to be adjusted in relation to the studied wastewater from different industries.
We studied the average daily flows of various breweries: Kharkov No. 1 and No. 2, Izyumsky, Kupyansky, Poltava, Melitopol and Belgorod.
The optimal conditions for the oxidation of wastewater with solutions of potassium dichromate were studied and an accelerated method for determining COD was proposed, according to which the analysis takes about 20 minutes, the consumption of sulfuric acid is 45 ml per water sample.
Considering that the results of COD determination by the accelerated method are somewhat lower than those obtained by the arbitration method, it was of interest to establish the relationship between the COD values found by the two methods, and thus make adjustments to the calculation when analyzing COD by the accelerated method.
COD was determined in wastewater samples using two methods. The relationship between COD indicators found by accelerated (X) and arbitration (y) methods, expressed in graphical form. To do this, in the general linear regression equation Y=a+bx determined the coefficients A And b solving a system of two equations:
| { | an + bΣx=Σy |
| aΣx + bΣx 2 =Σxy |
Where n- number of COD determinations.
It was found that a = -18.5; b= - 1.18 (or -1.2). Substituting these values into the general linear regression equation, we obtained an equation (see figure) relating the COD values determined by two methods:
y = 1.2x - 18.5.
To determine COD using the developed accelerated method, 5 ml of wastewater was pipetted into a 250 ml conical flask ( if the wastewater COD is higher than 600 mg O 2 /l, the wastewater was diluted 2 or more times with distilled water before analysis), added 5 ml of 0.1 N to the flask. solution of K 2 Cr 2 O 7 and while stirring, 15 ml of concentrated sulfuric acid was gradually added. After 2 minutes, the solution was cooled to room temperature, 50 ml of distilled water, 3-4 drops of an indicator (0.1% solution of phenylanthranilic acid) were added and titrated with 0.1 N. Mohr's salt solution.
The titer of the Mohr's salt solution was checked daily before analyzing the wastewater.
Simultaneously with the analysis of the prototype, a blank experiment was done, for which 5 ml of distilled water was taken and all stages of the analysis were carried out.
COD was determined using the formula:
COD = 1.2 · ((V 0 - V) · 0.1 · K · 8 · 1000/a) - 18.5
Where
V 0, V - respectively, the volumes of Mohr’s salt solution used for titration of blank and test samples, ml; 0,1 - normality of Mohr's salt solution;TO - correction factor for bringing Mohr's salt solution to 0.1 N;
8 - oxygen equivalent;
A - volume of analyzed wastewater, ml;
1.2 and 18.4 are coefficients for bringing the accelerated data to the indicators of the arbitration method for determining COD.
Graph of the relationship between the COD values of wastewater found by the accelerated (x) and arbitration (y) methods
|
Determined COD, mg O 2 /l |
x/y = z |
z 1 - z |
(z 1 - z) 2 |
|
|
developed method (X) |
arbitration method (y) |
|||
|
Σ =15,01 |
Σ =0,0853 |
|||
|
Note: z = Σ z/n=1 |
||||
To assess the accuracy of the developed method for determining COD using the data given in the table, we found:
S 2 = Σ (z 1 - z)2/(n- 1);
E= + t a S z
Erel = + E 100/2
Where n- number of COD determinations;
z - arithmetic mean of n definitions;
S 2 - sample variance of the method for a given number of determinations;
S z - root mean square error of the mean value;
a is the specified reliability;
t a is a multiplier that is found in special tables of mathematical statistics for the values of a and n;
E - accuracy of determination;
E rel - relative error of the method in %.
Value S z =0.061, E=0.044, Erel =4.4%. Thus, the developed method for determining COD in brewery wastewater is much faster than the arbitration method and requires less consumption of concentrated sulfuric acid. The relative error of the method is ±4.4%.
Private economy and industry generate a large amount of wastewater on the planet. This is why wastewater treatment facilities are so important. Thanks to modern methods of processing and disinfection of contaminated water, it is possible to reduce the level of threat to the environment, which, one way or another, exists due to the discharge of dirty liquid into water bodies.
The main indicators of water pollution, in accordance with which the treatment methodology is selected, are the calculation and analysis of COD (chemical oxygen demand) and calculation of the amount of BOD (biological oxygen demand) of water. It is by these parameters that the level of contamination of the liquid is determined and they strive to reduce it to the standards regulated by SNiP using specially selected disinfection methods.
Important: if the level of COD and BOD in industrial or private wastewater is several times higher, then the water poses a serious threat to the environment. Therefore, troubles with the environmental service cannot be avoided if the wastewater is not cleaned before discharge. Moreover, if even when water is disinfected, the levels of COD and BOD indicators do not fall during the calculation and analysis, it means that the technology for processing the liquid medium is broken.
During the natural self-purification of water, oxygen reactions occur, which allow the oxidation of organic impurities in the water. Thus, their partial or complete disintegration occurs. COD is an indicator of oxygen consumption for the oxidation of various impurities in water, and BOD is an indicator of oxygen consumption for the oxidation of impurities when interacting with bacterial aerobic preparations in wastewater treatment plants.
Thus, increased levels of COD and BOD when analyzed in wastewater indicate that the water requires a lot of oxygen to oxidize harmful impurities. This means that the amount of these same impurities is also large. That is, the water is too dirty.
COD and BOD levels are measured by taking water for analysis. In this case, water is examined at certain temperatures for a specific period of time.
During oxidation by oxygen in water, elements such as sulfur, hydrogen, carbon, phosphorus and other chemical components, excluding nitrogen, are destroyed to the state of CO2, H2O, P2O5, SO3. In addition, when participating in the oxidation of oxygen, nitrogen is converted into ammonium salt. It is worth noting that during the oxidation reaction, oxygen directly participates in the reaction, while hydrogen only donates three of its atoms to each oxidized atom of the substance. This is especially true for the oxidation of nitrogen and the formation of ammonium salts.
Important: The analysis for BOD in water takes longer from 5 to 20 days, and the analysis for determining COD takes from 0.3 to 1.4 days.
Reduced COD and BOD levels

Chemical and biological oxygen consumption levels in dirty water are reduced in special treatment plants. The principle of water purification is approximately the same. The only difference is in the method of influencing pathogenic microorganisms in order to maximize their destruction. At the same time, treatment plants can vary in design and size depending on the amount of wastewater processed and its initial formation.
To reduce the levels of chemical and biological (biochemical) oxygen indicators in a liquid, from 1 to 4 stages of processing are used. These are:
- Primary stage. It involves the mechanical separation of large particles of debris and fatty films by filtering or settling. Such methods are physical and mechanical.
- At the secondary stage Liquid disinfection uses biological preparations to oxidize smaller, sometimes dissolved organic impurities in water.
- During tertiary processing water neutralizes and removes metal salts and other remaining small particles of impurities. Here, chemical and physico-chemical processing methods are most often used, such as reverse osmosis, electrodialysis, adsorption, flotation, etc.
- Fourth stage Water treatment is not a method of reducing COD and BOD levels, but is aimed at separating (dehydrating) the residue remaining in the water and its subsequent disposal.
Important: most often when treating wastewater, the first two stages of water treatment are used. After this, the water contains normal indicators of biological and chemical oxygen consumption. In Europe, a third stage of liquid purification is sometimes used, but only when necessary.
Differences between industrial and domestic wastewater in terms of COD and BOD levels

Drains are divided according to the type of formation into industrial and domestic. Accordingly, the former contain more pollutants and chemical impurities, which require large amounts of chemical or biological oxygen absorption to purify them. In turn, household water is polluted primarily with organic matter, which forms several times lower levels of COD and BOD compared to industrial dirty water.
Important: if somehow household wastewater gets to industrial wastewater, then it is an activator of biological and biochemical oxygen absorption for liquid purification using one of the biochemical methods. That is, the quality and speed of water purification increases significantly.
Conversely, if aggressive substances such as chlorine are released into household wastewater or industrial wastewater is mixed into the water, this may indicate a high level of COD and BOD for household water.
Important: chemical oxygen demand in wastewater is measured in mg/liter. However, during analysis, the COD level will always be higher than the BOD level. Because chemical oxidation in water requires more oxygen than biological oxidation.