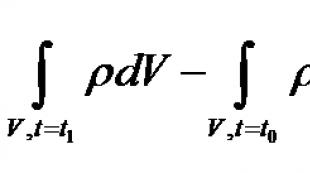Sorption methods of wastewater treatment. Sorption purification. Carbon-free sorbents for water purification
Sorption methods are the most common for the separation of chromium from wastewater from galvanic production. They can be divided into three types:
- 1) sorption on activated carbon (adsorption exchange);
- 2) sorption on ion exchangers (ion exchange);
- 3) combined method.
Adsorption method.
The adsorption method is one of the effective methods for extracting non-ferrous metals from electroplating wastewater. Activated carbons, synthetic sorbents, and industrial waste (ash, slag, sawdust, etc.) are used as sorbents.
Mineral sorbents - clays, silica gels, aluminum gels and metal hydroxides are little used for the adsorption of chromium from wastewater, since the energy of their interaction with water molecules is high - sometimes exceeding the adsorption energy.
The most versatile of the adsorbents are activated carbons, but they must have certain properties:
- - weakly interact with water molecules and good
- - with organic substances;
- - be relatively large-porous;
- - have a high adsorption capacity;
- - have low holding capacity during regeneration;
- - have high strength;
- - have high wettability;
- - have low catalytic activity;
- - have a low cost.
The process of adsorption extraction of hexavalent chromium from wastewater is carried out with intensive mixing of the adsorbent with the solution, by filtering the solution through an adsorbent layer or in a fluidized bed in batch and continuous installations. When mixing the adsorbent with the solution, activated carbon is used in the form of particles with a diameter of 0.1 mm or less. The process is carried out in one or several stages.
A number of researchers have studied the adsorption of chromium on activated carbon as a function of pH.
It has been established that chromium (VI) is easily adsorbed on activated carbon in the form of anions such as HCrO4 - and CrO4 2-. A number of studies have shown that pre-treatment of adsorbents with nitric acid increases their sorption capacity for chromium (VI).
There is a known method for the adsorption of chromium from wastewater using solid lignin. It was found that the sorption process depends on the pH of the solution and the dose of lignin. The optimal contact time of the solution with lignin is 1 hour. Activated carbon is mainly used as a sorbent; other sorbents are used extremely rarely. The following are suggested as other sorbents in various studies:
- a) waste from the brewing industry (cardboard with a sorbed strain of yeast Saccharomyces carlsbergensis;
- b) sawdust, preferably pine, treated with a copolymer of monoethanolamine vinyl ether with 4-methylazahepta-3,5-diene-1,6-diol vinyl ether (SVEMVE);
- c) plant material (sludge-lignin, cellulose, etc.);
- d) iron filings;
- e) zeolites, silica gels, bentonite;
- f) clays;
- g) vermiculite.
Advantages of the method
- 1) Cleaning to maximum permissible concentration.
- 2) Possibility of joint removal of impurities of different nature.
- 3) No secondary pollution of treated waters.
- 4) Possibility of recovery of sorbed substances.
- 5) Possibility of returning purified water after pH adjustment.
Disadvantages of the method
- 1) High cost and scarcity of sorbents.
- 2) Natural sorbents are applicable for a limited range of impurities and their concentrations.
- 3) Cumbersome equipment.
- 4) High consumption of reagents for the regeneration of sorbents.
- 5) Generation of secondary waste requiring additional treatment.
Ion exchange method.
Ion exchange extraction of metals from wastewater allows the recovery of valuable substances with a high degree of recovery. Ion exchange is the process of interaction of a solution with a solid phase that has the ability to exchange ions contained in it with ions present in the solution. The substances that make up this solid phase are called ionites. The ion exchange method is based on the use of cation exchangers and anion exchangers that absorb cations and anions of dissolved salts from the treated wastewater. During the filtration process, exchangeable cations and anions are replaced by cations and anions extracted from the wastewater. This leads to depletion of the exchange capacity of materials and the need for their regeneration.
Synthetic ion-exchange resins - high-molecular compounds whose hydrocarbon radicals form a spatial network with ion-exchange functional groups fixed on it - have acquired the greatest practical importance for wastewater treatment. The spatial hydrocarbon network is called the matrix, and the exchanging ions are called counterions. Each counterion is paired with oppositely charged ions, called anchor ions. The ion exchange reaction proceeds as follows:
RH + NaCL = RNa + HCL,
upon contact with cation exchange resin,
where R is a matrix with fixed ions; H - counterion,
ROH + NaCL = RCL + NaOH,
upon contact with anion exchanger.
To extract trivalent chromium cations from galvanic production wastewater, H-cation exchangers are used; chromate ions CrO32- and dichromate ions Cr2O72- are extracted using anion exchangers AV-17, AN-18P, AN-25, AM-p, AM-8. The capacity of anion exchangers for chromium does not depend on the pH value in the range from 1 to 6 and decreases significantly with increasing pH above 6.
At a concentration of hexavalent chromium in solution from 800 to 1400 eq/l, the exchange capacity of the AV-17 anion exchanger is 270 - 376 mol*eq/m 3 .
Regeneration of strong basic anion exchangers is carried out with an 8 - 10% solution of sodium hydroxide. Eluates containing 40 - 50 g/l of hexavalent chromium can be used for the production of sodium monochromate, and the purified water can be reused.
On the basis of VlSU, a technology for local treatment of chromium-containing wastewater has been developed in order to extract compounds of heavy non-ferrous metals from them, incl. and chromium by sorption on a strong base anion exchanger. The degree of water purification using this technology is more than 90 - 95%. Purified water complies with GOST 9.317-90 and is quite suitable for use in closed water circulation systems.
Manufactured: filters of the "ECOS-2" type in VNIIHT, sorbents: in the Scientific and Technical Center "MIUSORB" (Vidnoye, Moscow region), MP "Poisk" (Ashgabat), LLP "TET" (Dolgoprudny, Moscow region), VNIIHT (Moscow).
The company Inovan Umwelttechnik GmbH & Co KG has developed a block-modular installation of the REMA system, designed for the purification of industrial wastewater from heavy metals. A single block is an ion exchange column in which 4 replaceable cassettes are installed vertically one below the other. During the treatment process, wastewater is sequentially passed through these cassettes from bottom to top.
The degree of contamination of the ion exchange resin is determined using indicators.
The Pochvomash plant (Kirov) has introduced a process for purifying industrial wastewater from electroplating industries from chromium ions using fibrous materials. For the sorption of chromium anions, the material VION AS-1 is used, which contains strongly basic vinylpyridinium groups with a COE of 1.1 - 1.2 mg*eq/g. Two sorption columns were made of corrosion-resistant steel with a volume of 50 l each. The sorption of chromium depends on its concentration in the initial solution. So, if the concentration is up to 10 mg/l, then it is not detected in the filtrate. However, when the concentration of chromium anion is 75 mg/l and higher, its content in the filtrate is 0.04 - 0.01 mg/l, which is quite acceptable in a closed cycle. The effect of the initial concentration of chromium solution on its content in the filtrate is due to the high ionic radius of Cr2O72-, which causes steric hindrance during sorption on the fibrous chemisorbent. If the chromium content is high, the solution feed rate to the sorption column should be reduced. In this case, the degree of purification increases. When saturation of the sorption columns is achieved, they are removed from the stand and transported to the galvanochemical processing department for regeneration of the chemisorption material and disposal of the eluate. Regeneration of VION AS-1 is carried out with a solution of Na2CO3. In this case, 50 liters of solution are poured into each column and left for 3 hours. The next step is to wash the filter with water.
A study was carried out on 8 fibrous sorbents used to purify wastewater from heavy metal ions (Ag, Hg, Cr, Cd, Fe). It was found that fibrous sorbents PAN-PEA, PAN-TTO-MKKHK and carbon fiber effectively purify wastewater from heavy metal ions. They are easily regenerated by treatment with acids and can be reused for cleaning purposes. Metals can be isolated from the solution obtained after fiber regeneration and reused.
Ion-exchange materials based on waste from sewing and knitting production, containing polyester and polyacrylonitrile fiber, have been synthesized.
It has been established that the synthesized ion-exchange fibers exhibit selective ion-exchange properties.
In laboratory conditions, the separation of chromium from the washing wastewater of galvanic shops was studied using ion exchange resins (ion exchange resins in the OH form of the "Wolfatit" type (Germany) grades SWB, SZ, SL, SBK, AD-41 and activated carbon grade AS) and carbonaceous sorbents
The mod-ix system from Krebs & Co.AG (Germany) includes a pre-filter, valves, pipelines, pumps, devices for monitoring water quality by its electrical resistance and two integrated ion exchange columns with a throughput of 1.5 - 4 m 3 / h . One of the columns is used for its intended purpose, the other is being regenerated at this time. The described system consists of separate modules and is therefore easy to install and dismantle.
Advantages of the method
- 1) Possibility of cleaning to MPC requirements.
- 2) Return of purified water up to 95% into circulation.
- 3) Possibility of recycling heavy metals.
- 4) Possibility of purification in the presence of effective ligands.
Disadvantages of the method
- 1) The need for preliminary treatment of wastewater from oils, surfactants, solvents, organics, suspended solids.
- 2) High consumption of reagents for the regeneration of ion exchangers and resin processing.
- 3) The need for preliminary separation of wash water from concentrates.
- 4) Cumbersome equipment, high cost of resins
- 5) Formation of secondary waste eluates requiring additional processing.
Sorption (wastewater treatment)
Sorption is the process of absorption of a substance from the environment by a solid or liquid. Distinguish three types sorption:
-absorption– volumetric absorption of a substance by the entire mass of liquid or gaseous sorbent;
-adsorption– surface absorption of a substance by the surface layer of a solid or liquid sorbent;
-chemisorption– sorption, accompanied by chemical interaction of the sorbent with the absorbed substance.
Sorption is one of the most effective methods for deep purification of wastewater from various enterprises from dissolved organic substances industries industries: pulp and paper, chemical, petrochemical, textile and others.
Sorption method applies for extraction from wastewater valuable dissolved substances (phenol, arsenic, hydrogen sulfide) with their subsequent recycling and the use of treated wastewater in systems negotiable water supply
Sorption purification can be used on one's own and together with biological cleaning. Benefits The method is the possibility of adsorption of substances from multicomponent mixtures, as well as high efficiency of purification, especially of weakly concentrated wastewater.
Adsorption– the process of physical adhesion of molecules to the surface of a solid (adsorbent) without a chemical reaction occurring. Adsorption used for deep purification of closed water consumption and post-treatment of wastewater from organic substances, including biologically hard ones.
Adsorption methods widely apply for deep purification of wastewater from dissolved organic substances after biochemical treatment, as well as in local installations, if the concentration of these substances in the water is low and they do not decompose biologically or are highly toxic.
Adsorption use for neutralization of wastewater from phenols, herbicides, pesticides, aromatic nitro compounds, surfactant dyes, etc. Dignity The method is highly efficient, the ability to treat wastewater containing several substances, as well as the recovery of these substances.
Adsorption carried out as follows ways:
Add to waste water sorbent in crushed form, the resulting mixture is stirred, then settled and filtered;
Wastewater is continuously passed through filter, loaded with sorbent.
Adsorbents, used for water purification, are crushed powdery materials or granules with a diameter of 0.5 - 1 mm. They are added to the water that is in the clarifier - in this case, coagulation is combined with adsorption. Granular adsorbents are used in an apparatus having a device similar to a pressure filter.
As sorbents use various artificial and natural porous materials : activated carbons, zeolites (aluminosilicates), ash, slag, coke breeze, peat, sawdust.
Activity sorbent is characterized by the amount of absorbed substance per unit volume or mass of the sorbent (kg/m3, kg/kg). Adsorption properties activated carbons to a large extent depend on the structure of the pores, their size, size distribution.
Macropores (0.1 – 0.2 µm) and transition pores (0.004 – 0.1) usually play the role of transport channels, and sorption The ability of activated carbons is determined mainly microporous structure (micropores have a size of less than 0.004 microns).
Most effective sorbents for extracting organic substances from aqueous solutions are active carbons coals of various brands. The porosity of coals is 60–75%, and the specific surface area is 400–900 m2/g.
Activated carbon is porous a substance consisting of carbon with a small amount of impurities that play a very important role in adsorption. Coal, formed as a result of heating various organic substances without air access, contains impurities resins which clog his pores. To remove resins and increase porosity, coal is subjected to a treatment called activation.
Depending on the conditions of this treatment, activated carbon can adsorb predominantly acids or, conversely, bases. Adsorbing property acids has carbon activated at a temperature of 900 o C. Coal heated to 450 - 500 o C, on the contrary, adsorbs well grounds and does not adsorb acids. This explained the fact that on the surface of coal during processing, surface compounds of oxides that are basic or acidic in nature are formed.
Activated coal has a number benefits before other sorbents:
Rigid porous structure;
Sufficient mechanical strength;
Chemical and thermal resistance;
Hydrophobicity;
The ability to adsorb many organic substances that are not removed during biological treatment. Such substances are found, for example, in wastewater from oil refineries. In this case, the adsorption method on activated carbon is the most reliable and cheapest.
Research wastewater treatment carried out using adsorption methods using active carbons show high efficiency wastewater treatment. Dose coal depends on its adsorption capacity, the type of pollutants in the incoming wastewater and the required treatment effect.
Wastewater treatment using activated carbon has its own peculiarities. Concentration organic substances in wastewater can be very high. In such a system, coal adsorbs from 0.2 to 0.4 kg of substance per 1 kg of own weight. After completion of the adsorption process, organic substances burn out in a regenerative oven. Coal restore and sent again for adsorption. Wherein losses carbon adsorbent is approximately 5%.
On factories arrange large installations, consisting of filters for wastewater treatment and equipment for carrying out regeneration.
For the treatment of industrial wastewater, they are increasingly used. non-carbon sorbents of natural and artificial origin. The use of sorbents based natural materials (clay rocks, zeolites and other materials) due to relatively low cost, availability, high sorption capacity, as well as ion-exchange properties of some of them.
In adsorption technology, in addition to coal, the so-called silica gel– dehydrated silicic acid gel, it adsorbs predominantly grounds.
Among mineral sorbents of natural origin, the most widespread are clayey rocks that usually contain materials with a regular structure. Recently, much attention has been paid to zeolites.
To obtain durable and water-resistant filter media materials from natural zeolites their warm up in ovens at a temperature of 1000 o C with sodium chloride and carbonate. When heated quickly, zeolites foaming, as a result of which their volume and porosity increase by 5...20 times. Natural zeolites are used in the form powders and filter materials for cleaning water from surfactants, aromatic compounds, dyes, pesticides, colloidal and bacterial impurities.
Pricenatural sorbents are tens of times lower than artificial ones, so they are usually do not regenerate. Pre-treatment can significantly increase the cost of natural sorbents. Therefore, the feasibility of their use is determined taking into account technological, environmental, economic and other factors.
There is currently industrial production synthetic porous materials, some of which are classified as adsorbents based on their physicochemical properties. The porous structure of polymers is achieved by introducing an inert solvent into the mixture of reagents during polymerization, after the removal of which a complex system of pores is formed.
Adsorption purification can be regenerative, i.e. with the extraction of the substance from the adsorbent and its disposal, and destructive, in which substances extracted from wastewater are destroyed along with the adsorbent. Regeneration sorbents - restoration of adsorption capacity is more often used for granular active coals due to their high cost. In this case, some of the adsorbed substances (up to 20%) are irreversibly held in his pores. Activity coals gradually from cycle to cycle decreases.
In cases where adsorbed substances do not represent utilitarian value, or the costs of their disposal exceed their value, use destructive sorbent regeneration technologies. Destructive regeneration of the sorbent is usually carried out thermal or chemical methods. When choosing a technology for using sorbents, it is necessary to take into account that in the structure costs cost for sorption purification sorbents is 30...35%.
The simplest adsorber is an embankment filter. Usually sorption installation consists of several parallel working sections, consisting of three to five sequentially located filters.
Adsorbers with motionless layer of sorbent (sorption filters) are structurally made open (non-pressure) and closed (pressure). Usually at least three adsorption apparatuses are installed, connected so that two operate in series, and the third can be turned off at flushing or regeneration.
Devices continuous actions can significantly reduce the volume of active carbon. Principle The operation of the devices is that the liquid being purified moves from bottom to top, and a dense layer of the reagent moves towards it under the influence of gravity or with the help of various mechanical devices.
For the adsorption removal of dissolved organic contaminants from water at high-capacity treatment plants, devices with suspended ( fluidized) layer of active carbon. This makes it possible to use adsorbent grains relatively small sizes (0.2 - 1 mm) with a developed external surface. The devices have countercurrent movement of interacting phases.
Efficiency adsorption purification reaches 80 – 95%.





































































Description of the presentation Sorption methods of water purification Physico-chemical methods of water treatment 1 according to slides
Sorption methods of water purification Physico-chemical methods of water treatment 1 Lecture
The role of adsorption methods of water purification Physico-chemical methods of water treatment Water purification is, as a rule, reduced to the transfer of the pollutants contained in it into the solid (less often into the gas) phase. The transfer of substances present in water in ionic form into the solid phase is achieved by converting them into slightly soluble compounds (chemical precipitation) or by coprecipitation (coagulation). However, if the water contains dissolved substances in molecular form (especially if they are non-polar or weakly polar), their removal requires the use of other methods, among which adsorption appears to be the most promising. Adsorption is the absorption of molecules of a substance dissolved in water by a solid insoluble body - an adsorbent. Absorption occurs due to physical sorption or chemisorption on the developed surface of the adsorbent. Physical sorption is based on the forces of intermolecular interaction. Chemisorption is based on absorption with the formation of chemical compounds on the surface of a solid with the participation of chemical reactions. Adsorbents are solid insoluble bodies with a developed surface (up to 1000 m 2 /g) due to high porosity.
Structure of activated carbons Physico-chemical methods of water treatment The most common adsorbents are active (activated) carbons of different brands. Activated carbons are porous carbon bodies, granular or powdery, having a large surface area. A heterogeneous mass consisting of graphite crystallites and amorphous carbon determines the unique porous structure of active carbons, as well as their adsorption and physical-mechanical properties. The porous structure of active carbons is characterized by the presence of a developed system of pores, which are classified by size as follows: Micropores are the smallest type of pores, comparable to the size of the adsorbed molecules. The specific surface area of micropores reaches 800–1000 m2/g. Mesopores are pores characterized by layer-by-layer filling of the surface with adsorbed molecules, ending with their filling by the mechanism of capillary condensation. The specific surface area of mesopores reaches 100–200 m2/g. Macropores are the largest type of pores, the specific surface of which usually does not exceed 0.2–0.5 m2/g. Macropores are not filled during the sorption process, but act as transport channels for delivering the substance to the surface of the pores that adsorb it. In accordance with the standards of the International Union of Pure and Applied Chemistry IUPAC, pores with a diameter of less than 0.4 nm are called submicropores, pores with a diameter from 0.4 to 2.0 nm are micropores, pores with a diameter from 1 to 50 nm are mesopores and more 50 nm – macropores. - micropores - with a size of up to 20 A, - mesopores - with a size of 20–500 A, - macropores - with a size of more than 500 A.
The role of adsorption methods of water purification. Physical and chemical methods of water treatment. The adsorption properties of active carbons are assessed by the amount of model substance sorbed by a unit mass of coal under certain conditions, as well as by the time of protective action of a unit volume of coal until it is completely saturated. Basically, the adsorption properties of carbons are determined by micropores, which constitute up to 90% of the entire surface of active carbon. Adsorption processes take place on it, which are based on the interaction of energetically unsaturated carbon atoms with molecules of adsorbed substances. Meso- and macropores perform mainly a transport role. A large volume of large pores leads to a decrease in the density of the adsorbent and its capacity. Substances are sorbed better in molecular form, worse in ionic form. The ability of organic substances to sorption increases in the series: glycols< спирты < кетоны < сложные эфиры < альдегиды < недиссоциированные кислоты < ароматические соединения. Способность к сорбции возрастает с ростом молекулярной массы и температуры.
Mechanisms of adsorption on coals. Physico-chemical methods of water treatment. Adsorption in micropores (specific volume 0.2 -0.6 cm 3 /g and 800 -1000 m 2 /g), comparable in size to the adsorbed molecules, is characterized mainly by a volumetric filling mechanism. Similarly, adsorption also occurs in supermicropores (specific volume 0.15 -0.2 cm 3 /g) - intermediate areas between micropores and mesopores. In this region, the properties of micropores gradually degenerate, and the properties of mesopores appear. The mechanism of adsorption in mesopores consists of the sequential formation of adsorption layers (polymolecular adsorption), which ends with the filling of the pores according to the mechanism of capillary condensation. For ordinary active carbons, the specific volume of mesopores is 0.02 -0.10 cm 3 /g, the specific surface is 20 -70 m 2 /g; however, for some active carbons (for example, brightening ones), these figures can reach 0.7 cm 3 /g and 200-450 m 2 /g, respectively. Macropores (specific volume and surface area, respectively, 0.2 -0.8 cm 3 /g and 0.5 -2.0 m 2 /g) serve as transport channels that bring molecules of absorbed substances to the adsorption space of activated carbon granules. Micro- and mesopores make up the largest part of the surface of activated carbons; accordingly, they make the greatest contribution to their adsorption properties.
Mechanisms of adsorption on coals. Physico-chemical methods of water treatment. Micropores are particularly well suited for the adsorption of small-sized molecules, while mesopores are particularly well suited for the adsorption of larger organic molecules. The determining influence on the pore structure of activated carbons is exerted by the feedstock from which they are produced. Activated carbons based on coconut shells are characterized by a larger proportion of micropores, and activated carbons based on coal are characterized by a larger proportion of mesopores. A large proportion of macropores is characteristic of wood-based activated carbons. In active carbon, as a rule, there are all types of pores, and the differential distribution curve of their volume by size has 2-3 maxima. Depending on the degree of development of supermicropores, activated carbons are distinguished with a narrow distribution (these pores are practically absent) and wide (substantially developed).
Mechanisms of adsorption on coals. Physico-chemical methods of water treatment. In the pores of activated carbon, there is intermolecular attraction, which leads to the emergence of adsorption forces (Van der Waals forces), which in nature are similar to the force of gravity with the only difference that they act at the molecular, and not at the astronomical level. These forces cause a reaction similar to a precipitation reaction, in which adsorbed substances can be removed from water or gas streams. Molecules of removed pollutants are held on the surface of activated carbon by intermolecular van der Waals forces. In this way, activated carbons remove contaminants from the substances being purified (unlike, for example, bleaching, when molecules of colored impurities are not removed, but are chemically converted into colorless molecules). Chemical reactions can also occur between the adsorbed substances and the surface of the activated carbon. These processes are called chemical adsorption or chemisorption, but basically the process of physical adsorption occurs through the interaction of activated carbon and the adsorbed substance. Chemisorption is widely used in industry for gas purification, degassing, metal separation, as well as in scientific research. Physical adsorption is reversible, that is, the adsorbed substances can be separated from the surface and returned to their original state under certain conditions. In chemisorption, the adsorbed substance is bound to the surface through chemical bonds, changing its chemical properties. Chemisorption is not reversible. Some substances are weakly adsorbed on the surface of ordinary activated carbons. These substances include ammonia, sulfur dioxide, mercury vapor, hydrogen sulfide, formaldehyde, chlorine and hydrogen cyanide. To effectively remove such substances, activated carbons impregnated with special chemicals are used. Impregnated activated carbons are used in specialized applications in air and water purification, in respirators, for military purposes, in the nuclear industry, etc.
The main options for using sorption methods of water purification. Physico-chemical methods of water treatment. Adsorption methods can be implemented in two main ways: 1) Filtration through a layer of granular activated carbon, 2) Dosing powdered activated carbon into the treated water (charcoalization of water), 3) Filtration through a fibrous material containing activated carbon. According to the shape and size of the particles, activated carbons can be powdery, granular (crushed and granulated), and also fibrous. Powdered ones have a particle size of less than 0.1 mm, granular ones - from 0.5 to 5 mm, fibrous ones - a diameter of less than 0.1 mm and a length of several centimeters. Powdered activated carbons are used for water purification once at waterworks, introducing them during or after coagulation. Granulated carbons are used for water purification by filtration in devices with a continuous layer of sorbent (mechanical filters). Depending on the type, coals can be regenerated with live steam or reagents. However, due to the complexity of organizing such a process, large losses of coal and the impossibility of its complete regeneration (only 40–70%), coal is usually used once for water purification. Fibrous activated carbons have the largest effective surface area and can be used in specially designed water filters. They have found application in household filters. To assess the quality of granular active carbons used as loading in various types of adsorbers, physical and mechanical characteristics are important, such as: fractional composition (graining), bulk density, mechanical strength.
Main characteristics of activated carbons. Physico-chemical methods of water treatment. Granulometric size (granulometry) is the size of the main part of active carbon granules. Unit of measurement: millimeters (mm), mesh USS (American) and mesh BSS (English). Bulk density is the mass of material that fills a unit volume under its own weight. The unit of measurement is gram per cubic centimeter (g/cm3). Surface area is the surface area of a solid divided by its mass. The unit of measurement is square meter per gram of coal (m 2 /g). Hardness (or strength) - all manufacturers and consumers of activated carbon use significantly different methods for determining strength. Most methods are based on the following principle: a sample of activated carbon is subjected to mechanical stress, and the measure of strength is the amount of fine fraction or medium-sized grinding produced during the destruction of coal. The amount of undestructed coal in percent (%) is taken as a measure of strength. Humidity is the amount of moisture contained in active carbon. The unit of measurement is percentage (%).
Main characteristics of activated carbons Physico-chemical methods of water treatment of the river. N of aqueous extract - p value. N of an aqueous solution after boiling a portion of active carbon in it. Protective action - measuring the time of adsorption of a certain gas by carbon before the minimum concentration of gas begins to pass through the layer of activated carbon. This test is used for coals used for air purification. Most often, activated carbon is tested for benzene or carbon tetrachloride (aka carbon tetrachloride CCl 4). CTC adsorption (adsorption on carbon tetrachloride) - carbon tetrachloride is passed through a volume of activated carbon, saturation occurs to a constant mass, then the amount of adsorbed steam is obtained, related to the sample of carbon in percent (%). Iodine index (iodine adsorption, iodine number) is the amount of iodine in milligrams that can be adsorbed by 1 gram of activated carbon, in powder form, from a dilute aqueous solution. Unit of measurement – mg/g. Methylene blue adsorption is the number of milligrams of methylene blue absorbed by one gram of activated carbon from an aqueous solution. Unit of measurement – mg/g. Molasses discoloration (molasses number or index, indicator for molasses) - the amount of activated carbon in milligrams required for 50% clarification of a standard molasses solution.
Production of activated carbons Physico-chemical methods of water treatment For the production of activated carbon, furnaces of various types and designs are used. The most widespread are: multi-shelf, shaft, horizontal and vertical rotary furnaces, as well as fluidized bed reactors. The basic properties of active carbons and, above all, the porous structure are determined by the type of initial carbon-containing raw material and the method of its processing. First, carbon-containing raw materials are crushed to a particle size of 3-5 cm, then subjected to carbonization (pyrolysis) - roasting at high temperature in an inert atmosphere without air access to remove volatile substances. At the carbonization stage, the framework of the future active carbon is formed - primary porosity and strength. However, the resulting carbonized carbon (carbonate) has poor adsorption properties because its pore sizes are small and the internal surface area is very small. Therefore, the carbonate is subjected to activation to obtain a specific pore structure and improve adsorption properties. The essence of the activation process is the opening of pores that are in a closed state in the carbon material. The basic principle of activation is that the carbon-containing material is subjected to selective heat treatment under appropriate conditions, as a result of which numerous pores, crevices and cracks are formed and the pore surface area per unit mass is increased. The technology uses chemical and steam-gas activation methods. There are two types of activation: chemical activation and gas activation.
Chemical activation Physico-chemical methods of water treatment During chemical activation, mainly non-carbonized source materials are used, which include peat and sawdust. Sludge waste from clarification processes can also be used. The transformation of such raw materials into activated carbon occurs under the influence of dehydrating agents at high temperatures. In this case, oxygen and hydrogen are selectively and completely removed from the carbon-containing material, while carbonization and activation occur simultaneously (usually at temperatures below 650°C). Carbonated materials have a reduced oxygen and hydrogen content, so they are not activated by inorganic agents as easily as non-carbonated materials. Phosphoric acid, zinc chloride and potassium sulfide are mainly used as activating agents in technology. Activation with phosphoric acid can be carried out according to the following scheme: finely ground raw materials are mixed with a solution of phosphoric acid, the mixture is dried and heated in a rotary kiln to 400 -600 ° C. There are known processes that are carried out at higher temperatures (up to 1100°C). To obtain wide-porous coals, used primarily for clarification, a significantly larger amount of phosphoric acid is required than in the production of coals for gas purification and water treatment.
Chemical activation Physicochemical methods of water treatment When activated with zinc chloride, 0.4–5 parts in the form of a concentrated solution are mixed with 1 part of the raw material, the mixture is heated to 600–700°C. The advantages of this activation method are undoubtedly the relatively short activation time of the starting materials, the high yield of carbon residue, and the good adsorption properties of activated carbon. Typically, chemical activation produces soft and powdery products. Mixing carbon-containing raw materials with a carbon-containing binder (for example, sawdust with lignium sulfonate) and an activating agent and subsequent molding produces durable activated carbon. Chemical activation of coals in a rotary kiln for 3 hours using phosphoric acid and zinc chloride as activating additives makes it possible to obtain molded products that are not inferior in strength to steam-activated coals.
Activation with water vapor and gases Physico-chemical methods of water treatment When processing carbon-containing substances with oxidizing gases under appropriate conditions, part of the carbon burns out and is removed with volatile components and the internal surface increases. The oxidizing agents used are mainly water vapor, carbon dioxide and oxygen or air. Care must be taken when using oxygen because it reacts with carbon 100 times faster than carbon dioxide. When carbon reacts with water vapor or carbon dioxide, the following reactions occur simultaneously: Since these are endothermic reactions, heat is required. In this case, good heat exchange between the reactivating gas and the coal particles is crucial. This requirement is met by the constant movement of coal particles during the activation process in rotary kilns or fluidized bed reactors. When using steam, a temperature of about 800°C is required to ensure an effective reaction rate, and when using carbon dioxide - 900°C. If heat is supplied mainly by the activating gas, its temperature should be even higher.
Activating furnaces Physico-chemical methods of water treatment Activation of carbon-containing materials with oxidizing gases is carried out at a sufficient speed only at temperatures of 600 -1000°C. As noted, the reaction of the solid material with the activating gases used in production (usually water vapor and carbon dioxide) is endothermic. Accordingly, a constant supply of heat is required. On the other hand, the subsequent combustion of these gases is accompanied by the release of energy. Thus, reactors used in technology for gas activation must have the following conditions: 1) heating the reaction material to a high temperature; 2) good contact between the carbon-containing substance and the activating gases; 3) supply of heat necessary for the reaction; 4) possible lower consumption of thermal energy of the reaction gas. The following types of furnaces used in production meet these conditions: rotary, shaft, multi-shelf, fluidized and moving bed reactors.
Rotary kilns Physico-chemical methods of water treatment. Rotary kilns can be used to activate fine and granular or shaped products. Contact between the carbonaceous material and the activating gases can be improved by using mixing devices. The activation time depends on the angle of the oven, as well as the presence of internal partitions and the size of the support rings. The activated material and gas can be supplied in one direction or in countercurrent. In addition, there are two designs: stoves with internal and external heating. Internally heated rotary kilns are equipped at the top, where the carbon material is charged, with a burner fed by liquid fuel or gas. The inner surface of the furnace is lined with refractory bricks. Rotary kiln: 1 – lifting blades along the length of the kiln; 2 – furnace masonry; 3 – burner.
Shaft furnaces Physico-chemical methods of water treatment Shaft furnaces consist mainly of chambers located vertically one above the other, the walls of which are lined with refractory bricks. The activated material is loaded from above, and water vapor is supplied from below. The use of nozzles or guide devices allows you to increase the reaction surface and improve mixing. Shaft furnace: 1 – channel for supplying reaction gases; 2 – fire channel. Shaft furnaces are used to activate lump coal, which is then processed into granular or powdered coal.
Fluidized bed reactors Physico-chemical methods of water treatment In fluidized bed reactors, the activated products and gases are thoroughly mixed. This significantly reduces activation time. The simple design of a fluidized bed reactor consists of a sealed cylindrical or rectangular reaction chamber equipped with a perforated distribution grid at the bottom through which reaction gases enter. The process can be continuous or batch. Multistage reactors are known, consisting of vertically and horizontally located chambers with transitions between them, as well as reactors consisting of a large number of compartments separated by partitions. They are designed to activate fine-grained and, in some cases, shaped coal. The process can be improved by heating the internal volume of the reactor with the heat obtained during combustion and generated during the activation process with water vapor. Another possibility for additional heat input and increased productivity is to externally heat the reactor. Fluidized bed reactor for gas activation: 1 – “quiet” volume; 2 – fluidized bed level; 3 – external heating; 4 – heat exchanger; 5 – distribution grid; 6 – reactor. The figure shows a diagram of a furnace into which heated activating gases are supplied at a speed that ensures immobility of the lower layer and fluidization of the upper layer of the charge. This creates the possibility of soft activation of various raw materials.
Adsorption methods of water deodorization Physicochemical methods of water treatment Non-polar adsorbents are widely used in the practice of drinking water preparation to extract organic substances from them that cause tastes and odors. When adsorption of organic impurities from solutions, preference is given to activated carbons, since water (solvent), characterized by a high surface tension at the interface with the surface of coal grains, is adsorbed negligibly. The dose of coal during static adsorption is determined by the formula: where C 0 and C f are, respectively, the concentrations of the adsorbed substance before and after adsorption, T is the specific adsorption in mg/l at the point corresponding to C f. The rate of adsorption of organic substances from water depends on the structure of coal, the specific surface area of granules (grains), conditions of mass transfer with the treated water and river. N water. If several substances are present in a solution at the same time, adsorption proceeds according to the law of displacement. As the number of substances removed from water increases, the proportion of adsorption of each of them decreases. The degree of adsorbability of various substances from water is estimated by the decrease in free energy ΔF ads
Dependence of ΔF adc on classes of organic substances during adsorption on coal KAD iodine from aqueous solutions Physico-chemical methods of water treatment Along with coalification (static conditions), water deodorization at stations of various capacities is carried out using stationary adsorbers under dynamic conditions - by filtering the source water through a layer of granular coal with a grain diameter of 1-2 mm and a thickness of up to 2.0 m. A distinction is made between the dynamic loading capacity E d (mg-eq/g) of the adsorber (before the adsorbed substance begins to leak into the filtrate) and the total E total. (mg-equiv/g) after the cessation of extraction of the adsorbed substance from water. No. Substances ΔF adc 1 phenol 5, 07 2 benzenesulfonol 4, 83 3 chloral hydrate 3, 26 4 formic acid 4, 21 5 oxalic acid 3, 22 6 naphthalene 5, 85 7 chloroform 4, 83 8 dichloroethane 4,
Parameters of the water carbonization process Physico-chemical methods of water treatment In the absence of odorous substances of biological origin during adsorption on coals of various brands (BAU, CAD, etc.), differing in pore size, the intensity of the water odor decreases significantly with increasing dose of active carbon from 2 to 20 -35 mg/l at r. H = 4 -12 and water temperature from +6 to +35°C. The main role in the adsorption capacity of coals is played by micropores with a radius of (1.1 -2.5) · 10 -7 mm with a specific surface area of up to 1000 m 2 /h. When coaling water, coals that are easily wetted by water should be used. The advantage of this method is the small required capital costs, while the disadvantages are the wasteful consumption of expensive adsorbent and the complexity of operation. It must be taken into account that fine coal powder with air forms an explosive mixture, and the volume of the room for its storage is required in the size of 2 -4.5 m 3 /t.
Parameters of the water carbonization process Physical and chemical methods of water treatment Depending on the adsorption capacity of active carbons and the intensity of water pollution with substances that give it unpleasant tastes and odors, coal consumption can vary within a very wide range - from a tenth of a milligram to 1000 mg/l. The most used doses of coal for coalification of natural waters are in the range of 3 -15 mg/l. Thus, when deodorizing water contaminated with substances that create tastes and odors of biological origin, their complete elimination with the help of OU-A sch coal was achieved at doses of 10 -12 mg/l. In practice, the charcoalization process includes the operations of soaking pulverized coal, creating a coal suspension with a coal content of up to 2.5-5% and dosing it into the treated water. Activated carbon is administered 10-15 minutes before introducing other reagents. The required contact time of the adsorbent with the treated water is at least 15-20 minutes. At the initial stage of water treatment with its primary chlorination, a powdered sorbent is introduced before or after the introduction of chlorine, depending on the interaction of chlorine with substances that create tastes and odors.
Sorption materials and their properties Physico-chemical methods of water treatment
Sorption materials and their properties. Physico-chemical methods of water treatment. In water treatment technology, activated carbon is used in the form of powder when carbonating water, crushed or uncrushed granules when filtering through carbon filters. To purify water from contaminants, dry dosing of powdered activated carbons, wet dosing (in the form of a suspension), filtration through a suspended layer of activated carbon, filtration in stationary adsorbers with granular activated carbon, filtration through combined sand-carbon filters are used. The choice of brand of adsorption material consists of selecting the parameters of its porous structure depending on the size of the molecules of the adsorbed substances. If there is one substance with low molecular weight in water, for example, phenol, ammonium nitrogen, nitrite nitrogen, then these substances, having a relatively low molecular weight and molecular size m = 0.63 nm, are best sorbed in micropores (m< 0, 63 -0, 7 нм) и супермикропорах (0, 6 -0, 7 < т < 1, 5 -1, 6 нм). Для этого случая пригодны активированные угли, имеющие требуемую структуру пор, типа АГ-3 и МАУ-100. Если в воде находятся нефтепродукты, СПАВ, гуминовые кислоты (по отдельности или смесь), то данные вещества, имеющие более крупные размеры молекул (т ~ 1, 8 нм), лучше всего сорбируются в мезопорах (1, 5 -1, 6 < т < 100 -200 нм). В этом случае пригодны активированные угли и сорбенты, имеющие требуемую структуру пор, например, мезопористый сорбент СГН-30. Если в воде присутствует смесь низко- и высокомолекулярных соединений (нефтепродукты, СПАВ, азот аммонийный, азот нитритный), то данные вещества, имеющие различные размеры молекул наиболее полно будут сорбироваться на адсорбентах, имеющих хорошо развитую структуру микропор и мезопор (таких как АГ-3, МАУ-100).
Designs of adsorbers and the basis of their calculations Physico-chemical methods of water treatment Design of an adsorber with a suspended layer of adsorbent 1 - counterflow of purified water and adsorbent (water moves from bottom to top, and adsorbent from top to bottom); 2 - collection of purified water; 3 - removal of purified water: 4 - supply of source water; 5 — adsorbent supply; b - removal of coal pulp; 7 - distribution system of purified water. Design of a stationary adsorber 1 - layer of activated carbon; 2 - supporting layer; 3 - source water pipeline 4 - pipeline for filtrate removal; 5 — filter housing; 6 - drainage system; 7 - reflector
Designs of adsorbers and the basis for their calculations Physico-chemical methods of water treatment The height of the required layer of coal loading is determined by the formula: where Vр. f. — design filtration speed, taken equal to 10 -15 m/h; τ y is the time of passage of water through a layer of coal, taken equal to 10 -15 minutes depending on the sorption properties of coal, the concentration and type of water contaminants and other factors and clarified by technological research. The duration of operation of the adsorption layer of the filter until the appearance of an adsorbed substance in the filtered stream with a concentration C pr exceeding the maximum permissible, τ pr and the length of the adsorbent layer L are related in the classical equation of sorption dynamics, proposed for calculations by N. A. Shilov: where τ pr - time before “breakthrough” - time of the protective action of the adsorbent layer, min; L—height of the adsorbent layer, cm; τ 0 and k are constants: τ 0 =h/ν - characterizes the space and time necessary for the formation and conduct of the mass transfer process; k = A 0 /(C 0 *ν) - protective action coefficient, min/cm; ν—liquid flow rate, cm/min; A 0 is the maximum dynamic capacity of the adsorbent at a given initial concentration of CO; h is the “dead” layer, a mathematical function characterizing the unused length of the adsorbent layer, see
Calculations of adsorption parameters. Physico-chemical methods of water treatment. The adsorption process, which takes place under dynamic conditions, consists of a period of formation of the adsorption front, characterized by a variable: the speed of its advance and a period of its parallel transfer at a constant speed. The dependence of the protective effect of the layer τ pr on its length L is graphically described by the OAB curve (Fig.). The stage corresponding to the formation of the adsorption front corresponds to the OA curve. Starting from the values expressed by the OL 0 section, the protective effect of the filter layer depends on its length (the second period of the dynamic adsorption process). The quantities k, τ 0, and L 0 are determined graphically: k = tan ﮮ BHL, L 0 = OL 0, τ 0 = OD and h = OH. Regeneration of the filter sorption load is carried out with a 5% Na solution. OH or by calcining coal at a temperature of 700 -750 ° C in the absence of air. Dependence of the time of protective action on the thickness of the adsorbent layer. The dose of the sorbent for each substance is determined by the formula: where C i k is the required final concentration of the substance, mg/l; a is the maximum amount of adsorbed substance, mg/mg, determined from adsorption isotherms. Based on analytical data, the following values of a can be accepted: for substances that determine the color of water - 0.046 deg/mg; for easily oxidized organics (permanganate oxidability) - 0.0086 mg 0 2 /mg; for difficult-to-oxidize organics (COD) - 0.02 mg 0 2 /mg; for ammonium nitrogen (NH 4) - 0.00066 mg/mg; for phenols - 0.002 mg/mg; for pesticides - 0.04 mg/mg; for chloroform - O, 16 mg/mg.
Calculations of adsorption parameters. Physico-chemical methods of water treatment. The total dose of the sorbent is determined by the formula: where k η is a coefficient that takes into account the degree of use of the equilibrium static adsorption capacity of the sorbent granules, taken equal to 1.2 -1.3. The mass of the sorbent introduced into the OSF is determined by the formula: where D Ʃ is the total dose sorbent, mg/l; Q in - water flow; T work - duration of the filter cycle, hours. Filtration is carried out with an upward flow of the treated water. The filter cycle stops when a controlled water quality indicator begins to “break through” into the filtrate. The average duration of the filter cycle is usually 12 -14 hours, after which the load is washed with a reverse flow of clean water for 3 -4 minutes with an intensity of 12 -15 l/ (s m 2). The floating load expands during washing (up to 40-50%). The grains of the adsorption material move downward under the influence of gravity and are discharged from the filter housing into a special container through a system of shut-off and control valves.
Physico-chemical methods of water treatment The problem of odor in tap water and the technology of dosing powdered activated carbons at water stations in St. Petersburg
Physico-chemical methods of water treatment Drinking water quality standards in Japan No. Indicator Standard value 1 Total microbial number Not more than 100 CFU in 1 ml 2 Total coliform bacteria Should not be detected 3 Chloroform Not more than 0.06 mg/l 4 Aluminum Not more than 0.2 mg/l 5 Iron Not more than 0.3 mg/l 6 Geosmin Not more than 0.00001 mg/l 7 2-methylisoborneol (MIB) Not more than 0.00001 mg/l 8 Total organic carbon (TOC) Not more than 5 mg/l l 9 p value. H 5, 8 – 8, 6 10 Color No more than 5 degrees
Physicochemical methods of water treatment Kinetics of sorption of odorants by coals of different grades Y 1 - 2 -isopropyl-3 -methoxypyrazine, Y 2 - 2 -isobutyl-3 -methoxypyrazine, Y 3 -2 -methylisoborneol, Y 4 -2, 4, 6 -trichloroanisole , Y 5 — geosmin Silcarbon TH 90 G OU-A Carbopal MB 4 Ebadaya LG 20 S Silcarbon TH 90 G
Kinetics of sorption of odorants by coals of different grades Y 1 - 2 -isopropyl-3 -methoxypyrazine, Y 2 - 2 -isobutyl-3 -methoxypyrazine, Y 3 -2 -methylisoborneol, Y 4 -2, 4, 6 -trichloroanisole, Y 5 - geosmin Physico-chemical methods of water treatment Hydraffin SC 14 FF HRMS
Results of pilot tests to study the effect of activated carbon on filtration parameters in the process of contact coagulation 12 -13. 05 Physico-chemical methods of water treatment
Changes in filtrate turbidity and increase in pressure losses during filter cycles Physico-chemical methods of water treatment
Installation for the preparation and dosing of solutions from dry material KD 440 from ALLDOS Physico-chemical methods of water treatment Characteristics of PAH Hydraffin S
Results of production tests and analytical determinations of water samples of raw and purified water of the Air Force during the pilot industrial operation of the PAH dosing plant 08. 2005 – 06.09.2005. Physico-chemical methods of water treatment. The duration of the filter cycle (interval between washings) of the cleaning unit during production tests averaged 12 hours, the same as without the use of PAHs. In this case, the average turbidity of the filtrate was 0.26 mg/dm3, color value was 5.2 degrees. , oxidability - 2.9 mg/dm 3, r. N – 6.5, and the content of residual aluminum in water is 0.09 mg/dm 3, which fully complies with the requirements of the San. Pi. N 2. 1. 4. 10. According to the results of analytical support for the pilot operation of a PAH dosing unit, carried out at the Research Center for Electrical Biology of the Russian Academy of Sciences, it follows that the content of petroleum products in purified water during the dosing period of PAH OU-A decreased compared to their content in raw water by 2.4 times, during the dosing period of PAH Hydraffin SC 14 FF - by 2.1 times. Permanganate oxidation of purified water when using PAH OU-A decreased by 64.4% compared to its value in raw water, when dosing PAH Hydraffin SC 14 FF – by 64.0%, while in the period without dosing PAH this figure was 56.3%. The bacteriological indicators of the filtrate for the entire test cycle did not exceed existing standards.
Results of production tests and analytical determinations of water samples of raw and purified water of the Air Force during the pilot industrial operation of the PAH dosing plant 08. 2005 – 06.09.2005. Physico-chemical methods of water treatment. Date dose PAH OC-A äî çà Hydraffin SC 14 FF mg/l 1 rise 2 MO% removal 1 rise 2 MO 01. Aug—8, 803, 8056, 820, 03—-02. Aug—7, 203, 2055, 560, 04—-03. Aug—8, 203, 2060, 980, 110, 01—-04. Aug—8, 503, 7056, 470, 01—-05. Aug—9, 104, 2053, 850, 01—-08. Aug—7, 203, 4052, 780, 04—-09. Aug 3, 00 -0, 220, 089, 403, 3064, 890, 03 ——10. Aug 5, 00 -0, 340, 058, 803, 1064, 770, 02 ——11. Aug 5, 00 -0, 540, 147, 502, 8062, 670, 02 ——12. Aug 5, 00 -0, 180, 067, 002, 6062, 860, 04 —— Aug 15, 7, 00*) -0, 070, 848, 302, 7067, 470, 05 —— Aug 16, 7, 00 *)-0, 070, 267, 202, 6063, 890, 04 ——17. Aug 7, 00 -0, 380, 097, 502, 6065, 330, 05 —— Aug 18, 7, 00*) -0, 097, 002, 4065, 710, 02 —— Aug 19, 7, 00*) -0, 310, 268, 202, 8065, 850, 03 —— Aug 22 5, 00*) -0, 080, 138, 502, 8067, 060, 080, 01 —— Aug 23 5, 00*) -0, 340, 117, 402, 8062, 160, 120, 01 —- 24. Aug 3, 00*) -0, 060, 018, 202, 8065, 850, 060, 01 —- 25. Aug 3, 00 *)—7, 502, 8062, 670, 01——26. Aug—-8, 303, 5057, 830, 03——29. Aug-3, 000, 150, 087, 702, 7064, 940, 150, 080, 377, 007, 804, 1030. Aug-3, 000, 040, 067, 702, 8063, 640, 060, 223, 507, 803, 7031. Aug-5, 000, 090, 028, 502, 9065, 880, 090, 020, 596, 008, 003, 8001. Sep-5, 000, 050, 017, 403, 0059, 460, 050, 010, 237, 407, 903, 7002. Sep-7, 000, 040, 018, 202, 9064, 630, 040, 010, 577, 608, 003, 5005. Sep-0, 147, 408, 404, 4006. Sep—0, 577, 608, 403, 90 0, 180, 147, 912, 8464, 790, 050, 030, 406, 307, 903, 76 0, 220, 187, 922, 7864, 880, 050, 01 — - 0, 070, 047, 902, 8663, 710, 070, 040, 406, 307, 903, 76 —8, 173, 5856, 07 —0, 367, 508, 404, 15 **) Ï ÀÓ í å äî Meaning Wed. meaning for the dosing period of Hydraffin SC 14 FF total organic carbon according to CIKV data Change in the content of petroleum products and permanganate oxidation during the use of PAHs at the Air Force petroleum products according to CIKV data *) Ó-À (ï ðî èçî äñòî Î ÀÎ “SÑî ðáåí ò”, G. P åðì ü) chloroform according to CIKV data Avg. meaning for the period without dosing PAHs, petroleum products according to NICEB RAS data; permanganate oxidation according to CIKV data Avg. meaning during the PAUSr dosing period. meaning during the OU-A dosing period
Types of anthropogenic pollution and their main properties Physico-chemical methods of water treatment
Composition and performance of water treatment facilities of the YuVS The station has its own water intake. It is possible to receive raw water to the treatment plant from the pumping station of the 1st rise of the Northern Waterworks. The station includes: Two pump rooms of the 1st lift, design capacity: 1 n. O. – 745 t. m 3 / day. 2 n. O. – 625 t. m 3 / day. The main technological process of water treatment is carried out in five parallel operating units: two units of filtering and settling facilities (FOS-1 and FOS-2) and three units of contact clarifiers (BKO-1, BKO-2, BKO-3). Parameter Unit of measurement FOS-1 FOS-2 BKO-1 BKO-2 BKO-3 K-6 Design capacity thousand m 3 /day. 180 260 310 250 350 Year of commissioning 1933 1947 1966 1980 1990 11. 2010 *) Given capacity 99 99 230 182 184 - Four pump rooms of the 2nd lift, design capacity: 2 n. O. – 220 t. m 3 / day. 3 n. O. – 350 t. m 3 / day. 4 n. O. – 430 t. m 3 / day. 5 n. O. – 550 t. m 3 /day. *) Block K-6 reached full capacity in May 2011. Eight clean water reservoirs with a total volume of 113,000 m3
Technology of water treatment in the Southern Eastern Europe Main reagent and reagent-free technological processes used in water treatment: Sorption of organic pollutants using powdered activated carbon Two-stage disinfection (primary chlorammoniation of water using sodium hypochlorite and ammonium sulfate, water treatment in UV units before supplying water to the consumer ) Coagulation of pollutants Flocculation of solid-phase particles Water treatment is carried out at treatment facilities operating according to a single-stage (contact clarifier units, BKO) or two-stage (filter settling facilities, FOS) schemes: A single-stage scheme (BKO) includes: purification from mechanical impurities on drums grids; mixing reagents in mixers - narrowing devices; contact coagulation in a sand-loaded environment using contact clarifiers, combined with filtration. A two-stage scheme (FOS) includes: mixing water with reagents in corridor-type mixer channels; settling in horizontal settling tanks; filtration on fast filters through granular media (quartz sand).
Installation for the preparation and dosing of powdered activated carbon (PAC)
BLOCK K-6 Southern Waterworks of St. Petersburg main technological building, including a clarification unit, a filtration unit, an ozonator
General characteristics of the K-6 treatment plant complex for the production of drinking water The new K-6 complex of the Southern Waterworks is designed to produce a nominal daily volume of water equal to 350,000 m 3 /day with an operating mode of 24 hours. The complex produces drinking water of the required quality at any flow rate with productivity from 20% to 125% of the nominal The quality of purified water complies with - Russian drinking water standards: Sanitary norms. Pin - European drinking water standards: EEC Standard The new complex uses new modern technologies and equipment - pre-ozonation - filters with double-layer sand/activated granular carbon - sludge dewatering
Two-layer fast filters with loading (sand / granular activated carbon) At the K-6 block of the Southern Waterworks, a highly efficient sorption water treatment system is used using 1200 tons of AC and a purified water capacity of up to 350 thousand tons per day. Over the past two years, the KhTMIST department and its partners have completed and are in the process of concluding 6 contracts related to the processes of sorption water purification at the facilities of the State Unitary Enterprise “Vodokanal of St. Petersburg”.
Main parameters of filter structures Design flow rate 370,000 m 3 /day. = 15,417 m 3 /hour = 4.28 m 3 /sec Max. flow rate 462,500 m 3 /day. = 19,217 m 3 /hour = 5.35 m 3 /sec Number of filters 20 Filtration area of one filter 105.6 m 2 Total filtration area (20 filters) 2112 m 2 Sand layer - sand layer depth 0.6 m - uniformity coefficient 1, 4 - useful diameter of sand grains 0, 5 -0, 6 - volume of sand per 1 filter 63, 36 m 3 - total volume of sand (20 filters) 1267, 2 m 3 Layer of granular activated carbon - depth of GAC layer 1, 2 m - uniformity coefficient 1.4 - useful size 0.9 -1.1 mm - volume of GAC per 1 filter 126.72 m 3 - total volume of GAC (20 filters) 2534 m 3 Calculated values of filtration rate: Filtration rate at design flow rate 7 , 3 m/hour Filtration speed at design flow rate with one idle filter 7.7 m/hour Filtration speed at maximum flow rate 9.1 m/hour Filtration speed at maximum flow rate with one idle filter 9.6 m/hour Backwashing of filters Water consumption for backwashing 20 m/hour – 35 m/hour Air washing speed 30 – 50 m/hour Backwashing of filters is carried out in two stages: First stage – Air washing for 2-3 minutes. The consumption of supplied compressed air is from 30 to 50 m 3 /hour per 1 m 2 of filter layer. Second stage - Backwashing with water at a speed of 20 to 35 m 3 / m 2 / hour, depending on the temperature of the raw water. The duration of the backwash is approximately 15 -20 minutes. The duration of the filter cycle between backwashes is approximately 24 -48 hours. Volume of backwash water per filter: Volume of backwash water at 20 m/h (105.6 × 20 / 60) = 704 m 3 Max. volume of water for backwash at 35 m3/hour (105.6 × 35 × 15 / 60) = 924 m 3 Average volume of water for backwash - 814 m 3, 800 m allowed
Distinctive features of the technological solution used for water purification at block K-6 are the elimination of preliminary chlorination of water, which makes it possible to further reduce the content of organochlorine compounds in drinking water (currently this figure is more than three times lower than regulatory requirements due to the use of technology ammoniation of water), - preliminary ozonation of water with small doses of ozone, ensuring surface oxidation of humic compounds and improving their subsequent coagulation, - settling of water after coagulation in a thin-layer plate settling tank-clarifier, providing more effective removal of suspended solids compared to traditional settling tanks, - filtration water on two-layer filters loaded with granular activated carbon and quartz sand, providing additional removal of dissolved organic compounds, including petroleum products.
Average quality indicators of water purified in November 2011 at block K-6 and at other blocks of the YuVS Quality indicator Neva Block K-6 Other blocks of the YuVS (total) Treatment efficiency, % Block K-6 Other blocks of the YuVS (total) Turbidity, mg/dm 3 2, 26 0, 28 0, 58 87, 6 74, 3 Color, deg. 37. 4 3. 55 5. 88 90. 5 84. 3 Oxidability, mg/dm 3 7. 17 2. 03 2. 61 76. 8 70. 2 residual. aluminum, mg/dm 3 0.06 0.21 97.6 *) 92.8 *) r. N 6, 62 6, 63 *) Calculated based on the amount of coagulant administered.
Spent activated carbon storage bins Spent activated carbon is removed from the filters using a mobile eductor system, using water as the driving force. The coal is transported as a liquefied slurry to two drainage silos located in the sludge treatment unit. The spent carbon is removed from each filter and replaced with clean carbon stored at the water treatment plant. The full cycle of the reactivation process lasts approximately a month, and includes unloading one of the filters, filling containers, transporting the spent coal, reactivating the carbon, transporting it back to the treatment plant in large plastic bags and storing until the next cycle.
Storage of granular activated carbon at block K-6 The storage of fresh granular activated carbon is located next to the sludge processing building. There are also silos for storing spent activated carbon. Both storage facilities are located on the ground floor level. Fresh or reclaimed coal, packaged in plastic bags, arrives at the facilities in a trailer and is stored in the sludge treatment building. The storage area for new or restored activated carbon is determined based on the storage of 126 m 3 of coal plus a 5% reserve for overload losses. To load one filter, 126 m 3 of activated carbon is required. Bunkers for spent activated carbon Bunker capacity (effective) 62 m 3 Bunker diameter 4 m Bunker height 7.5 m Distance under the bunker for delivery of containers 3 m Bunker material Fiberglass (fiberglass) Number of bunkers
Granulated carbon is transported to the filters using an ejector
System for loading activated carbon into the filter structures of block K-6 Ejector system for the hydraulic transport of fresh and regenerated carbon to the filters Basic calculation data: Volume of activated carbon in one filter 126.72 m 3 Linear speed in the diluted pulp pipeline 1.5 – 2.0 m /sec Pulp density 0. 12 kg coal / l water Pressure drop About 5 mbar / 1 m pipeline Consumption of activated carbon 9 m 3 / hour Estimated time for transporting coal to one filter 14 hours Ejector system: Manufacturer: Koerting, Hannover Type: Mobile jet ejector for transport of solid particles Number of units: 1 Weight: 38 kg Ejector system for hydraulic transport of waste coal from filters Linear speed in the diluted pulp pipeline 1. 5 – 2. 0 m/sec Pulp density 0. 12 kg coal / l water Drop pressure About 5 mbar / 1 m of pipeline Activated carbon consumption 15 m 3 / hour Estimated time for transporting coal from one filter 8. 5 hours Ejector system: Manufacturer Koerting, Hannover Type Rigidly fixed jet ejector for transport of solid particles Number of units 1 Weight 38 kg Both pressure pipelines for transporting GAC are laid parallel to each other: from the filter gallery, between the filter block and the reagent facilities, and ends in the sludge processing block. Transportation of spent GAC from a separate filter is carried out using technical driving water, which is pumped by a GAC transportation pump located in the process water pumping station. Water is distributed through the filter gallery to each filter (the filters are connected through flexible hoses to a portable GAC ejector), and then, through the ejector and the external pipeline for transporting spent GAC, coal is supplied to the spent GAC bunkers.
Characteristics of GAC Filtrasorb TL 830 GAC brand Filtrasorb TL 830 is a carbon of increased strength, ensured by the use of special binders in its production. The special manufacturing technology determines the relatively high price of GAC Filtrasorb TL 830. An important feature of the K-6 block, which determines the efficiency of its operation, is the need to maintain at the required level the sorption capacity of GAC, used as a load (together with quartz sand) in the rapid filters of the block. Bulk density, no more than 430 g/dm 3. Density of the dry product (true density), 1.2 g/cm 3. Humidity, no more than 2.0%. Total ash content, no more than 10.0%. Granule size d eq. = 0.9 -1.1 mm Mechanical strength (abrasion), not less than 75%. Porosity (pore volume) total, not less than 1.0 cm 3 /g. Specific surface area according to BET, 950 m 2 /g Clarifying ability for methylene blue - not less than 200 mg/g Adsorption activity for iodine, not less than 1000 mg/g. Sorption characteristics of GAC Filtrasorb TL-
Research of the state of the Filtrasorb TL 830 GAU during its operation at the K-6 block. According to the technology supplier organization, the service life of the Filtrasorb TL 830 GAC before reactivation is 3 years. After this period, the technology supplier (TAHAL, Israel) recommends reactivating the GAC. As a result of the work carried out by specialists from the Department of Chemical Technology of Materials and Sorption Equipment Products, it was established that the state of the GAU Filtrasorb TL 830 loaded into the unit’s rapid filters differs significantly from the initial state of fresh coal. A decrease in the sorption activity of GAC during its operation at the K-6 YuVS block was established. The rate of decrease in the sorption activity of GAC Filtrasorb TL 830 under the conditions of its operation at the K-6 YuVS block is: - for methylene blue - 4.25 mg/g per month, - for iodine - 25 mg/g per month, - for permanganate oxidation - 0.0175 mg/g per month. The work performed showed that by the summer of 2013, the sorption activity of GAC loaded into the rapid filters of block K-6 will be less than 25% of the initial values. A decrease in the sorption activity of GAC Filtrasorb TL 830 to values constituting less than 20% of the initial sorption activity of coal will lead to its irreversible loss, since reactivation and further exploitation of coal will become impossible. In this case, it will be necessary to completely replace the used sorbent with a fresh one, which, as shown below, will lead to economic damage for the enterprise SUE Vodokanal of St. Petersburg.
Ways to preserve the functional state of the rapid filters of the K-6 block In the course of carrying out the work, specialists from the department of “Chemical technology of materials and products of sorption equipment” considered three options for preserving the functional state of the rapid filters of the K-6 block: 1) Carrying out sequential reactivation of the GAC by step-by-step unloading from the existing ambulances filters and its reactivation at a specialized industrial enterprise. At the same time, it must be ensured that the productivity of the K-6 block for the treated water is maintained (at the same time it is necessary to unload and reactivate the GAC from only one rapid filter). 2) Overloading of filter structures with quartz sand (transition to the use of single-layer loading and refusal to use the sorption method of water purification). At the same time, it must be ensured that the productivity of the K-6 block for the treated water is maintained (at the same time it is necessary to overload the GAC in only one fast filter). 3) Overloading the upper layer of the filter structures (GAC) with fresh granulated carbon Filtrasorb TL 830. At the same time, it must be ensured that the productivity of the K-6 block for the treated water is maintained (at the same time, it is necessary to replace the GAC with quartz sand in only one fast filter).
The reactivation process involves four thermal steps: * Drying at 100°C: removing water. * Thermal evaporation at 100 -250°C: physical desorption of adsorbed volatile organics. * Formation of carbonizate at 200 -750°C: pyrolysis of non-volatile organic matter and carbonization of pyrolysate. * Gasification of carbonate at 800 -1000°C: gasification of pyrolysate by controlled reaction with steam, carbon dioxide or oxygen. Reactivation is the return of spent coal to production with sufficient activity to be used in the process for which it was originally intended. Reactivation = Return to production by thermal reactivation Regeneration = Reuse by steam treatment or chemical regeneration at the point of use. Reactivation of activated carbon consists of: Unloading carbon from the adsorber Treatment in a special furnace at high temperatures Replenishment of losses Reloading carbon into filters
Summary technical and economic results of options for organizing the operation of filter structures of the K-6 block after the depletion of the sorption resource of the GAC. According to the technology supplier organization, the service life of the GAC Filtrasorb TL 830 before reactivation is 3 years. After this period, the technology supplier (TAHAL, Israel) recommends reactivating the GAC. option for organizing the operation of filtration facilities amount of capital costs, thousand rubles. expected increase in operating costs, thousand rubles. expected increase in the cost of purified water, rub. /m 3 risk level *) Overload of the upper layer of rapid filters with fresh GAU 114 203, 61 **) - 0, 36 **) 2 Overload of the upper layer of rapid filters with quartz sand 23 919 634 158 865 0, 45 9 Overload of the upper layer of rapid filters reactivated GAU 68 163 800, 6 - 0.15 3 Notes: *) The level of risk is assessed on a comparative 10-point scale (0 - no risk, 10 - the strongest, unacceptable risk), **) The minimum size of the indicator for the acquisition of GAU in LLC is presented NPP "Polikhim". When purchasing GAU from other suppliers, the indicator will be higher.
Information about LLC NPP Polikhim NPP Polikhim is one of the leading enterprises for the production of modified carbon sorbents in the North-West of Russia. The enterprise has the following divisions: 1. Shop for the production of carbon sorbents 2. Shop for the production of equipment from plastic 3. Section for anti-corrosion protection of steel equipment 4. Design department 5. Design department 6. Estimation department 7. Research laboratory 8. Installation section and commissioning. The annual production output is currently 600 tons/year at the nominal productivity of electric furnaces. The same furnaces can be used to reactivate activated carbons. ← Electric furnace EVP-300 in the production building of LLC NPP "Polikhim" Electric furnace EVP-300 M in the production building of LLC NPP "Polikhim" →The company has its own production and produces granular activated carbons for fine water purification under the brands MAU-200, MAU-3 PT, MAU-6 A. The technology for producing active carbons of these grades was developed with the direct participation of the St. Petersburg State Technological Institute (Technical University), in particular, the department of “Chemical technology of materials and products of sorption equipment”.
Conducting a trial reactivation of GAC Filtrasorb TL 830 at the production facilities of LLC NPP Polikhim Granular activated carbons can be easily regenerated at LLC NPP Polikhim using the most common standard method (steam) Carrying out the process of reactivating coal with steam in the EVP-300 electric furnace Reactivated carbon (in as a result of reactivation in the EVP-300 oven). In accordance with the terms of the agreement between the State Unitary Enterprise Vodokanal of St. Petersburg and St. Petersburg. GTI (TU) at the production site of NPP Polikhim LLC in September-November 2012, a pilot industrial reactivation of spent GAC Filtrasorb TL-830 in an amount of 0.5 tons was carried out.
Results of the trial reactivation of GAC Filtrasorb TL 830 at the production facilities of NPP Polikhim LLC. Reactivation of spent GAC Filtrasorb TL-830 from fast filters of the K-6 YuVS unit allows you to restore the parameters of the GAC structure and even improve these parameters (with double reactivation) not only compared with the parameters of the waste material, but also in comparison with the parameters of the original fresh coal sample Filtrasorb TL-830 Ws - total pore volume, Vmi - micropore volume, Vme - mesopore volume. Sample Ws, cm 3 /g Vmi, cm 3 /g Vme, cm 3 /g TL-830 (lot 8613 E 008), original (fresh coal) 0.467 0.374 0.093 Sample of spent TL-830 (14 08.12) 0.433 0.359 0.074 Sample of reactivated TL-830 (14.08.12) 0.508 0.462 0.046 Batch of spent TL-830 (13.09.12) 0.403 0, 355 0, 048 Batch of reactivated TL-830 (09/13/12), single reactivation 0, 446 0, 420 0, 026 Batch of reactivated TL-830 (09/13/12), double reactivation 0, 547 0, 499 0, 048 The obtained results are explained by the fact that the manufacturer of GAC (Chemviron Carbon), in order to ensure a long overall service life of GAC Filtrasorb TL-830, based on the use of multiple processes of its reactivation, produces an underactivated product, thereby laying the possibility of preserving its sorption and operational properties properties during repeated reactivation.
Parameters of the porous structure of GAC Filtrasorb TL-830 samples before and after reactivation. The observed decrease in the mechanical strength of GAC during the reactivation process is associated with the removal of a small proportion of the binder component that occurs during the reactivation process. However, the mechanical strength of the reactivated samples of GAC Filtrasorb TL-830, amounting to 78 -80%, differs slightly from the mechanical strength of the original coal (84 -85%), which ensures the possibility of its further operation without any reduction in performance characteristics. Wо – measured volume of sorption space, Eо – adsorption energy for benzene. During the reactivation process, the sorption activity for methylene blue and the iodine number value are restored and even increase compared to the fresh GAC sample. sample W 0 , cm 3 /g E 0 , k. J/mol HP, mg/g Sorption activity by MG, mg/g Mech. strength, % TL-830 (lot 8613 E 008), original (fresh coal) 0. 378 25. 4 927 198 84 Sample of spent TL-830 (14.08.12) 0. 369 20. 8 759 98 80 Sample of reactivated TL-830 (14.08.12) 0.476 25.6 1080 213 78 Batch of used TL-830 (13.09.12) 0.369 20.2 689 94 85 Batch of reactivated TL-830 (13.09. 12), single reactivation 0.444 22.7 1016 211 80 Batch of reactivated TL-830 (13.09.12), double reactivation 0.509 26.
Conclusions based on the results of the research performed The optimal solution for organizing the operation of the filter structures of the K-6 block after the sorption resource of the GAC has been exhausted is to reactivate the coal in a third-party organization with its subsequent loading into the block’s rapid filters and reuse. NPP Polikhim LLC is recommended as a third-party organization to carry out the reactivation of spent coal from the rapid filters of the K-6 block. This organization is distinguished by 1) the high quality of the production process of GAC reactivation, established by conducting a trial reactivation of a batch of Filtrasorb TL-830 GAC, selected from the existing rapid filters of block K-6, 2) the lowest cost of reactivation among Russian enterprises of a similar profile, 3) the closest location in relation to the Southern Waterworks of St. Petersburg. The cost of a set of measures to reactivate spent gas from the rapid filters of block K-6 and reload the filters with reactivated carbon is approximately 68 million rubles. , which is almost 2 times lower than the capital costs of replacing used GAC with fresh coal. The implementation of a set of measures to reactivate spent GAC from the rapid filters of the K-6 block and reload the filters with reactivated carbon will be accompanied by the least significant increase in the cost of purified water at the K-6 block, amounting to 15 kopecks per 1 m 3, which is 2 times less than the same indicator , achieved when replacing spent GAC with fresh coal and is 3 times less than when replacing spent GAC with quartz sand. Replacing spent GAC from the rapid filters of the K-6 block with quartz sand is not recommended due to the inevitable sharp deterioration in the quality of water purification at the K-6 block and the associated economic and material damage for the State Unitary Enterprise Vodokanal of St. Petersburg.
Sorption methods of concentration are based on different absorption of dissolved substances, gases and vapors by solid or liquid absorbers ( sorbents). In contrast to coprecipitation, here absorption occurs already on the finished sorbent.
During the sorption process, the substance is distributed between two immiscible phases: solid - liquid, solid - gas, liquid - gas. In inorganic analysis, sorption is most often carried out in a solid-liquid system.
Based on the difference in the mechanism of interaction of the substance with the sorbent, there are physical(or molecular) sorption And chemisorption. At physical sorption interaction between the sorbent and the sorbed substance ( sorbitol) is caused by intermolecular forces, and there are adsorption(absorption of the substance by the surface of the sorbent) and absorption(absorption of the substance by the entire mass of the sorbent).
Chemisorption - This is an absorption based on the occurrence of chemical reactions between the sorbent and the sorbed substance with the formation of chemical compounds (ion exchange, complexation, oxidation-reduction, etc.). However, in practice it is difficult to encounter any of the sorption mechanisms in their pure form: they usually act in combination. Thus, adsorption usually precedes chemisorption. When concentrating microcomponents, adsorption and chemisorption methods have become most widespread, and among the latter are ion exchange and sorption accompanied by complex formation (for example, on chelating sorbents).
The sorption process can be carried out in various ways. From these positions they distinguish static, dynamic and chromatographic methods, and in each of them both sorption mechanisms are used.
Static(or single stage) The method is a one-time distribution of components between phases. When concentrating under static conditions, sorption of microcomponents is performed by conventional immersion of the sorbent in the sample solution. To speed up the achievement of equilibrium, the solution is stirred mechanically or using ultrasound. The concentration mechanism is molecular or chemisorption, in particular ion exchange, based on the exchange of solution ions for ions of the same charge sign included in the composition ion exchanger (ionite).
To separate the components of a mixture in a static way, it is necessary that they differ greatly in their ability to be distributed between phases and when equilibrium is established, some components are predominantly in one phase, while others are in another, i.e., the difference in the distribution coefficients of the separated components must be large. The distribution coefficient is defined as the ratio of the equilibrium concentration of a component in one phase (for example, in a solid) to the equilibrium concentration of the same component in another phase (for example, in a liquid):
Where D- distribution coefficient, Cj - equilibrium concentration of a component in one phase, C2 - equilibrium concentration of a component in another phase.
Sorption methods can be used to extract both microcomponents and the matrix. For the final determination of sorbed microcomponents, the sorbent is separated from the solution by decantation or filtration, and after washing (to remove foreign elements), the microcomponents are desorbed. Desorption is carried out similarly to sorption, using appropriate solvents. In some cases, before determining microcomponents, the sorbent is dissolved or ashed. But it is possible to analyze a sorbent containing microcomponents directly if methods such as X-ray fluorescence, neutron activation or atomic absorption with thermal atomization are used to determine them. Thus, in the case of X-ray fluorescence determination, it is enough to compress the sorbent with a concentrate of impurities into a tablet. The atomic absorption method with thermal atomization was used to determine trace amounts of Cd, Co, Cu, Ni, Pb, Zn in natural waters after their concentration on an ion exchanger (Dauex A-1 cation exchanger).
Dynamic and chromatographic methods are based on multiple distribution of system components between phases. Dynamic A variant of the selective concentration of microcomponents using the mechanisms of molecular sorption and chemisorption is carried out by filtering the solution of the analyzed sample through a thin layer of fine-grained sorbent applied to any substrate, a layer of paper with sorption properties, or a specially made membrane. This is a very simple concentration method, but its use is possible only in the case of significantly different distribution coefficients of the separated components and the rapid occurrence of the sorption process itself. To achieve maximum extraction of microcomponents, it is necessary to ensure a low filtration rate or repeat the operation several times until sorption equilibrium is achieved. Washing of the sorbent and desorption of microcomponents is carried out by filtering the corresponding solutions.
Chromatographic methods, also being multi-stage, they are used in cases where the distribution coefficients of the components of the mixture between two phases differ little, and therefore it is not possible to separate them in a single-stage method. Chromatography - This is a very common method of separating and concentrating substances, based on the difference in their distribution coefficients between two phases, one of which is stationary, and the other moves directionally relative to the first (along a column or a thin layer of a stationary phase).
Chromatography is characterized by the presence of a sufficiently large interface between phases and a dynamic method of performing separation. The combination of these two features makes chromatography a highly effective separation method, allowing one to separate substances that are very similar in their properties, even such as isotopes of elements or optically active isomers.
To concentrate microcomponents, various sorbents are used, which, along with good absorption capacity and selectivity, must be easily regenerated and be chemically and mechanically stable. From inorganic sorbents hydrated oxides, sulfides, phosphates of polyvalent metals are used, for example hydrated dioxides and phosphates of titanium, zirconium, tin and silicon, copper sulfide, salts of heteropolyacids. The mechanism of action of these substances is different, but most often sorption is due to ion exchange and complexation. The advantages of inorganic sorbents are resistance to heat, ionizing radiation, organic solvents, and often high selectivity. Among their disadvantages, we can note that the capacity is not always high enough and the sorption properties are poorly reproducible from batch to batch.
Inorganic sorbents were used to isolate microquantities of Ga, In, Ge, Mo, V, W and U from sea water (sorption on hydrated titanium oxide followed by analysis of the concentrate by the atomic emission method) and microquantities of P, As and W in the analysis of water and bottom deposits (sorption on aluminum oxide followed by determination by the neutron activation method), as well as for the extraction of mercury vapor from the air (sorption on lead sulfide and determination of mercury by the atomic emission method with a hollow iron cathode).
From organic sorbents Activated carbons, ordinary and modified cellulose, chelating sorbents, and synthetic ion exchangers have found widespread use.
Activated carbons have extremely developed micro- and macroporosity; they are obtained by burning wood or animal bones without access to air. On active carbons, molecular adsorption processes predominate (although sorption by other mechanisms, such as ion exchange, also plays a role). System includes adsorbent- a substance with a developed specific surface area and adsorbate - a substance whose molecules are absorbed. During adsorption, the substance is concentrated at the interface under the influence of molecular forces on the surface of the adsorbent. Physical adsorption is usually easily reversible. Activated carbon is used for concentrating silver and gold during their determination in rocks and ores, lead from air by filtration through a graphite disk with subsequent determination by the atomic absorption method, impurities of a number of metals (Ag, Bi, Cd, Co, Cu, Fe, In , Mn, Ni, Pb) contained in sodium perchlorate NaClQ* (in the latter case, the salt is dissolved in water and the resulting solution is filtered through a paper filter covered with a thin layer of active carbon; after desorption of impurities with concentrated nitric acid, they are determined by the atomic absorption method).
Very often, metal complexes with specially added reagents are sorbed on active carbons (chelates), which are selected so that the adsorption of the macrocomponent is significantly less than the adsorption of complexes of microcomponents. The efficiency of concentrating complex compounds is determined by stability constants, the nature of the complex and the structure of the ligands, and the charge of the complexing metal. This technique is carried out by introducing a complexing agent into the analyzed solution or directly fixing it on active carbon. Sorption of chelates on active carbons is one of the methods for group concentration of microimpurities in natural waters. Group concentration of Fe, Cu, Zn, Cd, Cr, Hg, Mn, Ni, Co, Pb, Re, etc. from natural waters is achieved by adsorption of microcomponents after converting them into chelates with dithizone, diphenylcarbazide, 8-hydroxyquinoline and subsequent filtration solution through a paper filter coated with active carbon. Microcomponents are absorbed quantitatively, the concentration coefficient is ~10 4 . In the resulting concentrate, impurities can be determined directly by atomic emission, X-ray fluorescence, neutron or 7-activation methods, or after their desorption with nitric acid by atomic absorption and photometric methods. When determining impurities (Ag, Cd, Co, Cu, In, Ni, Pb, Tl, Zn) in tungsten, they are concentrated on active carbon in the form of diethyldithiocarbamates, desorbed with nitric acid and determined by atomic absorption and X-ray fluorescence analysis.
In the practice of separating and concentrating elements, natural and synthetic polymers - high-molecular organic (or inorganic) compounds - are widely used as sorbents. Suitable for these purposes natural polymers should be called a polysaccharide cellulose(or plant fiber). Cotton is almost pure cellulose. Cellulose is used to concentrate Pt metals from dilute solutions. When determining microquantities of Cu, Fe, Mn and Zn in soil extracts, concentration is carried out by passing the analyzed solution through cotton wool. The same sorbent is used to concentrate traces of Cd, Cu and Pb in the atomic absorption analysis of drinking water, acetone and methanol. The method is simple, since cellulose filters with microcomponents sorbed on them can be analyzed by X-ray fluorescence without additional processing.
In practical analysis, sorbents based on polyurethane foams- highly porous materials with open pores and a very large specific surface area. Removal of sorbed impurities is achieved by dissolving the foam in hot nitric acid.
IN chromatographic method sorption, the most widely used sorbents are synthetic polymers of organic nature (resins), working on ion exchange mechanism (ion exchangers), i.e. the reason for sorption here is chemisorption. Ion exchangers that exchange with cations of the solution are called cation exchangers, and those exchanging with anions - anion exchangers. The chemical formulas of cation exchange and anion exchange are usually written as RH and ROH; then the cation exchange equation in general can be represented as a reaction:

where R is a complex organic radical (matrix or framework of a polymer), M",+ is a metal cation in oxidation state p+.
For an anion exchanger, the exchange process can be schematically written as
where A" is the anion of the basic acid.
A reversible stoichiometric equivalent exchange of ions from a solution to ions of the same sign that are part of the ion exchanger occurs when the analyzed electrolyte solution is passed through an ion exchanger placed in a long cylindrical glass tube (column version). The separation of ions is achieved due to their different affinities for the ion exchanger, as a result of which they move through the column at different speeds. Some components remain in the upper layer of the sorbent, others, which have a lesser degree of interaction with the sorbent, end up in the lower part of the column; still others leave the column along with the mobile phase. The result is a chromatogram - a regular distribution of substances into zones in accordance with their sorbability. Absorbed substances are removed from the sorbent by passing any suitable solvent through it ( eluent). In this case, the reverse process occurs - desorption, and the absorbed components return to the liquid phase ( eluate). The eluent must have selectivity to one or another ion in the mixture to be separated. By selecting the composition and acidity of the eluent, it is possible to successively remove all sorbed ions from the column. For this purpose, the ability of separated ions to form complexes of varying stability at a certain pH is often used.
Thus, gallium and lead can be sorbed together on an SBS cation exchanger column. If the column is washed with a 3 M solution of ammonium acetate, then lead ions, forming a soluble complex with acetate ions, pass into the eluate, and gallium remains on the column, from which it is then eluted with a 1.3 M solution of HC1. Quantitative determinations of the components after their separation can be performed by any suitable method.
The dynamism of the sorption process, ensured by multiple acts of sorption-desorption of the separated components in the flow of the mobile phase, determines the higher efficiency of the chromatographic method compared to sorption methods under static conditions and makes it possible to achieve fine separation of complex mixtures without much difficulty. At the same time, by sorption of substances from a large volume of solution and their desorption into a smaller volume of solvent, concentration sorbed substances.
The efficiency of separating two ions is characterized by separation factor, defined as a relation distribution coefficients for identical conditions of these two ions b’ = D/D 2 . If S= 1, then separation of ions is impossible. For separation it is necessary that the distribution coefficients D And D 2 were quite different.
The difference in the sorption behavior of the separated elements is often increased by converting one of the separated elements into an anionic complex, while the second element must remain in the form of a free (solvated) cation. An example is the separation of iron(III) and aluminum after adding ammonium thiocyanate to a solution, in excess of which only iron forms the thiocyanate complex 3 ~. The elements are easily separated using an anion exchanger. Organic complexants are especially often used for these purposes.
The separation coefficient is influenced by both chemical (pH of the solution, the nature of the separated ions and their concentration in the solution, the chemical composition of the ion exchanger, etc.) and purely physical factors (the rate of flow of the solution through the column, the grain size of the ion exchanger, the height of the column, the temperature of the solution, etc. .d.). The closer the conditions are to equilibrium, the more successful the separation is. Achieving equilibrium is facilitated by keeping the solution at a low speed. The rate of ion exchange between the sorbent and solution increases with decreasing ion exchanger grain size. However, too small particles of the ion exchanger make it difficult for the solution to pass through the column. Separation improves with increasing column height and increasing temperature.
Ion exchange chromatography is used primarily for purposes divisions, however, this method is also useful for absolute concentration traces of ions from very dilute solutions, especially aqueous-organic ones. By passing large volumes of dilute solutions through layers of ion exchanger and subsequent extraction of the absorbed substance with a small volume of solvent (eluent), it is possible to increase the concentration of the substance by 200-500 times. In analytical control, this technique is often used to concentrate impurities before their determination in ultrapure materials (for example, determination of impurities of many metals in ultrapure Ge, Ga, GaAs, As, ASCI3, etc. by atomic absorption analysis after their concentration on the KU-2 cation exchanger -8), when analyzing natural and industrial waters for the content of heavy metals, for the extraction of uranium and radioactive isotopes, as well as for the extraction of non-ferrous metals during the industrial production of rare earth elements (REE), including the processing of lanthanide ores.
The analytical and technological uses of ion exchange chromatography are diverse. This method is used to separate elements with very similar properties, such as rare earth elements, transuranic elements, twin elements (for example, Zr-Hf), cis-trans- isomeric complexes of Co and Pt remove interfering ions (purification of water and sugar solutions), and concentrate valuable microcomponents from natural and industrial waters. Ion exchange chromatography is widely used in the analysis of alloys, non-ferrous metal ores and products of their processing, waste containing trace elements, as well as wastewater from enterprises in order to develop effective methods for treating industrial discharges.
A limitation of the use of the ion exchange method is the need to carry out a lot of preparatory work using fairly large quantities of acids, alkalis and other reagents, as a result of which many foreign substances accumulate in the solution, interacting with ion exchangers and, in addition, affecting the acidity of the solution and the conditions of complex formation. Nevertheless, the method of ion exchange chromatography is being intensively developed and is constantly expanding the boundaries of application due to the creation of new, selectively acting ion exchangers and the development of new, especially automated, methods for separating and concentrating elements.
In method sediment chromatography To separate substances, the different solubilities of precipitates obtained as a result of the reaction between the separated ions and the precipitant are used. The solid precipitant is mixed with a finely ground carrier or the latter is impregnated with a solution of the precipitant, after which the mixture is placed in a column. When a solution of a mixture of separated ions is passed through a column, they are deposited on the carrier in the form of sparingly soluble precipitates in the order (from top to bottom) of increasing solubility. The least soluble compound is precipitated first (at the top of the column), followed by the next most soluble compound, etc. Successful separation of the mixture is achieved by repeating the process of precipitate formation and dissolution many times during chromatography. According to the sorption mechanism, sediment chromatography belongs to chemisorption chromatography. The processes that occur when passing through a column a solution containing a mixture of two substances AX and BX, reacting with the precipitant CY to form precipitates AY and BY, can be expressed by the scheme:

If sediments AY and BY are colored, then the location of sediments in the column can be determined visually and thus assess the qualitative composition of the analyzed solution. In the case of colorless sediments, the chromatogram is developed by introducing into the column after chromatography specific reagents that form colored compounds with the ions being determined. To quantify the components, use a graph of the length (height) of the zone versus the ion concentration.
When the sediment chromatogram is washed with a suitable solvent that dissolves some sediments well and does not dissolve others, individual zones are sequentially washed out of the column, resulting in a clear separation of the components of the mixture. By sorption of components from a large volume of the analyzed solution and their desorption into a smaller volume of solvent, concentration of the sorbed substances is achieved.
As carriers in sedimentary chromatography, pure highly dispersed substances are used that have good filtering ability and are indifferent to the precipitant and the chromatographed solution: aluminum oxide and hydroxide, silica gel, barium sulfate, starch, sand (silicon dioxide), etc. Precipitants are reagents that form with the separated substances. ions precipitate (characterized by different solubility) and are indifferent to the carrier; Moreover, a necessary condition is the sorbability of precipitants on the carrier. An example is the separation of Hg 2+, Bi 3+ and Pb 2+ ions by passing the solution through a column of aluminum oxide impregnated with a solution of potassium iodide. In this case, three zones are formed in the column: the upper red one, corresponding to the Hgl 2 compound, the second black one - VPz and the lower yellow one - RY 2. When washed with a solvent, the zones move down the column, and lead is detected in the first portions of the eluate, then bismuth and finally mercury.
In addition to separation, the method of sedimentary chromatography is used to purify substances and concentrate them. Thus, to purify cobalt or zinc from nickel impurities, a column containing active carbon with dimethylglyoxime (DMG) is used; in this case, cobalt or zinc passes into the filtrate, and nickel remains on the column, from which it is then extracted with a dilute solution of hydrochloric acid. In this case, the concentration of nickel increases hundreds of times.
Sediment chromatography is used to label alloys. The solution obtained by dissolving the alloy shavings is passed through a column; the chromatogram is compared with chromatograms of standard alloy samples.
Physical adsorption of the separated components of the mixture on the selected adsorbent is the basis adsorption(molecular) chromatography. Here the stationary phase is the solid adsorbent, and the mobile phase is the liquid ( solid-liquid chromatography, TLC) or gas (gas adsorption or gas-solid chromatography, GAS or GTX). The nature of absorption depends on the method of processing the adsorbent and the structure of its active surface, but most of all - on the nature of the adsorbed substance.
In TLC, the mixture under study, dissolved in a suitable solvent, is passed through a column filled with an adsorbent that is chemically inert to the components of the mixture and the solvent and has sufficient adsorption capacity (aluminum oxide, calcium oxide and carbonate, silica gel, activated carbon, zeolites (aluminosilicates of alkali and alkaline earth elements ), sucrose, cellulose, starch). The solvent must also be chemically inert to solutes and the adsorbent and free of impurities. Depending on the chemical nature of the separated substances and sorbents, water, alcohols, acetone, ethers, dioxane, benzene, toluene, etc. are used as solvents.
When the analyzed solution is passed through the column, the elements, due to their different adsorbability, are distributed in the column in steps. Those that are more strongly sorbed are retained in the upper layers of the sorbent, and those that are less sorbed are retained in the lower layers. Separation is achieved elution(by washing) the column with a suitable solvent ( eluent), while the individual components of the mixture move unevenly along the column. First of all, the less adsorbable component is washed out from the column, then the more adsorbed component, etc. By collecting the liquid flowing out of the column ( eluate) in separate portions, get the so-called liquid chromatogram. As a result, the complex mixture under study will be divided into a number of fractions containing individual components, further quantitative determination of which in the fractions is usually carried out by some physical and chemical method (photometric, polarographic, etc.).
TLC is used in quantitative analysis to separate interfering cations and to separate organic substances in the analysis of products from the food, perfume and pharmaceutical industries.
In GAC (or GTC), the column is filled with the same sorbents as in TLC. The main requirements for gas, which here is the mobile phase and is called carrier gas, are lower adsorbability and chemical inertness to the components of the mixture being separated. Air, nitrogen, hydrogen, carbon dioxide, argon, and helium are used as carrier gases (the last two are the most convenient).
The initial sample can be gaseous, liquid or solid, but a prerequisite is the preliminary transfer of the chromatographed substances into the gas phase. To do this, the analyzed sample is introduced into a special chamber ( dispenser), the temperature at which is sufficient for complete evaporation of all components of the sample. When the carrier gas moves along with the vapor of the substance along the adsorption column, the components of the mixture are absorbed by the adsorbent in accordance with the sorption properties. With further passage of the carrier gas, the absorbed substances begin to desorb stepwise and, leaving the column, enter the detector-analyzer - a device that allows you to record any physical and chemical property of a binary system (carrier gas - sample component) in order to establish the presence and quantitative content of each component of the mixture. The detector readings are usually converted into an electrical signal and transmitted to a recording or recording device, such as a tape potentiometer. The most common thermal conductivity detectors ( katharometers), ionization and electron capture.
A representative of ionization detectors is the argon detector, used to determine gases dissolved in metal. The sample under study is melted in a vacuum furnace in the presence of graphite. In this case, nitrogen is released in free form, oxygen is converted into CO monoxide, and hydrogen is released in free form and partially in the form of methane. The released gases are absorbed in a column with an adsorbent and then washed with argon. The sequence of gases washed out is: hydrogen, nitrogen, methane, carbon monoxide. The gases enter the detector, where the carrier gas (argon) is partially ionized under the influence of radioactive /3 radiation. The admixture of gases released from the metal affects the degree of argon ionization. The resulting ionization current, after amplification, is fed to a recording potentiometer. The resulting chromatogram is a series of peaks. The peak area is proportional to the amount of each component, and the time the peak appears at a constant operating mode of the device (constant temperature and velocity of the carrier gas) characterizes the nature of the component (Fig. 21). Identification of the substances under study is carried out by comparing the peak times found by

Rice. 21.1 - 1b; 2 - N2; 3 - CH4; 4 - sum of gases
certain conditions, for the components of the analyzed mixture and for the standard sample.
The difference in the distribution of the separated substances between two immiscible solvents underlies distribution chromatography (liquid - liquid chromatography). Partition chromatography can be carried out on a column, on paper or in a thin layer of adsorbent. In the column version, a column filled with a carrier - a solid, finely ground porous substance - is impregnated with a stationary solvent, which, adsorbed on the surface of the carrier, forms a surface liquid film on it. The mixture of substances to be separated, dissolved in a mobile solvent, is introduced into the column and, after the solution is absorbed in the upper part of the column, it is washed with a clean mobile solvent. During the washing process, there is a continuous redistribution of the substances of the mixture between two immiscible liquid phases. If the components of the mixture have even a slight difference in the distribution coefficients between the mobile and stationary phases, then they will move along the column at different speeds. The component that has the highest distribution coefficient has the highest speed 
(where C|Ju dv and Snepodv are the concentrations of the dissolved substance in the mobile and stationary phases, respectively); therefore, this component will be washed out from the column earlier than others and will end up in the first portions of the eluate. Component with the smallest D moves in the column at the lowest speed and ends up in the last portions of the eluate. If the column length is sufficient, complete separation of the mixture components occurs.
The requirements for stationary phase carriers are the same as in the case of adsorption chromatography. Mobile and stationary solvents are selected depending on the nature of the carrier and its polarity. If the carrier is a hydrophilic substance (silica gel, cellulose, starch, aluminum oxide, etc.), then the stationary solvent is water or other polar liquids (sulfuric acid, methyl alcohol, nitromethane), and the mobile solvent is a less polar organic solvent or a mixture of solvents (for example, a mixture of butyl alcohol and chloroform). For example, uranium(U1) is separated from most other elements on silica gel treated with 6 M HNO3. Ypana(VI) salts are eluted from the column with methyl isobutyl ketone (MIBK). Metal ions that do not interact with MIBK remain on the column, from which they are then removed with an aqueous solution.
If the carrier is a hydrophobic substance (fluoroplastic-4, or Teflon), polystyrene and other polymers), then non-polar organic solvents (benzene, chloroform, kerosene, etc.) are used as stationary media, and polar organic compounds and water are used as mobile media . In this case the method is called reverse phase partition chromatography, or extraction chromatography. This type of chromatography gives good results in the separation of substances that are highly soluble in organic solvents.
This method is used to separate, for example, traces of iron from aluminum on a column filled with fluoroplastic-4 powder in the form of a suspension in tributyl phosphate (TBP). When passing a 7 M solution of HC1 through the column and then washing the column with a HC1 solution of the same concentration, iron is completely retained by the organic phase, and aluminum is quantitatively washed off the column under these conditions. The iron is then washed away
0.1 M HC1 and is determined photometrically with sulfosalicylic acid.
For group concentration of impurities when analyzing water and CaCl2 solution, a 0.1 M solution of 8-hydroxyquinoline in a mixture of carbon tetrachloride and isoamyl alcohol is applied to the fluoroplastic powder. After passing through the analyzed solution, the impurities are eluted with hot 2 M HCl. After evaporation of the eluate and mineralization of the residue, impurities are determined by the atomic emission method.
Extraction chromatography successfully separates inorganic ions in the form of complexes with organic ligands. Partition chromatography, which combines the extraction chemistry of the process with the chromatographic technique of its implementation, is one of the most effective methods of liquid chromatography. It allows you to separate almost any mixture of substances with similar chemical properties, since the number of combinations of pairs of separating liquids is unlimited.
Specific types of partition chromatography that make it possible to do without a column are paper And thin-layer chromatography, used to separate very small quantities of substances. In the first case, specially treated chromatographic paper serves as an inert carrier. When separating water-soluble substances, the stationary phase is water adsorbed by the carrier, and the mobile phase is the organic solvent. If the substances are soluble in an organic solvent, then water is used as a mobile phase, and the organic solvent is used as a stationary phase.
Mixtures of substances are most often used as solvents, for example, butyl or amyl alcohol with methyl or ethyl alcohol, saturated aqueous solutions of phenol, cresol, etc., mixtures of butyl alcohol with acetic acid, ammonia, etc.
To separate, a drop of the analyzed solution (containing 0.01-0.1 mg of the separated components) is applied to the edge of a strip of chromatography paper, which, after the drop has dried, is immersed in a vessel (cylinder) with a mobile solvent. In this case, the solvent level should be below the applied
174 Chapter 3. Methods for separating and concentrating elements
drops. Under the action of capillary forces, the solvent moves up the paper; upon reaching the area where the applied drop of solution is located, its components begin to redistribute between the mobile and stationary phases in accordance with the value of the distribution coefficients D. The more D(Spodv > Sniodv), the more the component passes into the mobile phase and moves with the solvent at a higher speed. After a few hours, when the mobile solvent rises to a sufficient height, the initially taken mixture will be divided into separate spots of components located at different distances from the place where the initial drop of solution was applied (starting line) (Fig. 22).
If the substances being separated are uncolored, then to “develop” the chromatogram in order to detect the separated components, reagents are applied to the areas where they are located, forming colored compounds with them.
To find the zones where each of the components of the analyzed solution is concentrated, use the relationship between the height of rise / of a given component and the height of rise L solvent front: ![]()

Rice. 22. Paper chromatography.
Rf- speed of movement of a component in a given system where Rf characterizes the speed of movement of a component in a given system and depends on the nature of the substance, properties of solvents, temperature, type of chromatographic paper, and does not depend (in an ideal case) on the concentration of the substance and the presence of other components. At constant experimental conditions (for given phases, carrier and temperature) Rf is constant and can serve as an individual characteristic of a component used to identify it. Usually Rf established for these conditions in preliminary experiments with pure components. So, for example, for the Fe 3+ ion Rf= 0.40 if the solvent is a mixture of ethyl and isobutyl alcohols with hydrochloric acid, and Rf= 0.10, if the solvent is a mixture of dioxane, antipyrine and hydrochloric acid.
Using the relation I = Rf? L, possible by measuring L, identify areas where the relevant components are concentrated and where their detection should be carried out. The separation of components is better, the more significantly they differ Rf. Condition for separating two ions: R t - R/2 ^0,1.
Quantitative determination of separated components is most often carried out by photometric or fluorimetric (luminescent) methods or, after elution of chromatographic spots, by the polarographic method.
In addition to the described so-called ascending method of paper chromatography, there are a number of its varieties (descending, radial, two-dimensional). The simplicity of the experiment and the variety of experimental conditions allow the use of paper chromatography in almost all areas of chemical research. This method is most widely used in biochemistry, for example, for the separation of amino acids and peptides when studying the structure of proteins, the separation of alkaloids, steroids and various extracts from natural products. In inorganic analysis, the method is used mainly in qualitative analysis for the separation and detection of rare earth elements and radioisotopes. Methods have been proposed that make it possible to detect and determine metals in soils and geological samples under field conditions.
Thin layer chromatography (TLC) is similar to paper chromatography in many ways. Separation here is carried out on plates (glass, plastic, metal) coated with a thin layer of sorbent with high adsorption capacity (silica gel, aluminum oxide, cellulose powder, polyamide, etc.). To fix the sorbent on the plate, it is mixed with a binding material (starch, gypsum). The lower edge of the plate with the sample applied to it is lowered into a mobile solvent. After completion of the separation process, the identification and quantification of the components of the analyzed mixture is carried out in the same way as in the case of paper chromatography.
The advantage of TLC over paper chromatography is the rapidity of the process (no more than 1 hour, and sometimes 10-15 minutes), the clarity of separation of components and the ability to separate and identify very small quantities of mixtures of substances - from several tens of micrograms to hundredths, and sometimes thousandths of a microgram . The method is simple to perform and in terms of equipment and is unsurpassed in the analysis of complex mixtures, which has led to its widespread use for the separation of molecular compounds in chemical, biological, medical and other studies.
In analytical control, TLC is used as a method for separating and concentrating substances in the analysis of various natural and industrial microobjects from 10 -5 -10~ b g samples (minerals, inclusions, semiconductor products, etc.). This method successfully separates rare and rare earth elements, platinum metals, and uranium fission products.
Partition chromatography is widely used for the analysis of gases, low- and high-boiling organic and inorganic mixtures (with a boiling point from -200 to 400 °C). The stationary phase here is a liquid deposited in a thin layer on the surface of any solid inert carrier, and the mobile phase is the carrier gas. gas-liquid chromatography(GLC). Inert substances with a developed surface but low microporosity are used as solid carriers to prevent gas adsorption on the surface. The most widely used carrier materials are kaolin, tripoli, Teflon, ceramics (various brands of highly porous bricks), specially prepared silica gel, purified starch, and cellulose. Carriers are also made from aluminum oxide, carborundum (silicon carbide SiC) or from tiny glass beads (balls).
The efficiency of separation in GLC depends mainly on the correct choice of the liquid phase, and therefore it is subject to a number of stringent requirements: the liquid phase must be firmly held on the solid carrier, be chemically inert to the components of the mixture and to the solid carrier, thermally stable, not dissolve the carrier gas, have low viscosity, be non-volatile and have fairly high selectivity. Polar and non-polar hydrocarbons, vaseline, silicone and high-boiling aviation oil, phthalates, high-vacuum grease and a number of other high-molecular organic liquids are used as a liquid phase in GLC. Speakers are made of glass, stainless steel, copper or aluminum.
The analyzed sample (liquid or gas) is quickly injected into the chamber in front of the column using a special dispenser. The temperature in this chamber is higher than in the column, due to which the liquid sample evaporates almost instantly and, entrained by the carrier gas, enters the column. A constant high temperature is maintained in the column using a thermostat, selected based on data on the boiling points of the components being determined and their thermal stability. Typically this temperature is slightly above the boiling point of the highest boiling component in the mixture being analyzed. When a carrier gas is passed through a column, multiple processes of dissolution and separation of the components of the gas mixture occur in it due to their different solubilities in the liquid film. Therefore, they move at different speeds along the height of the column and, therefore, appear at the output at different times. At the exit, the gas jet passes through a detector, with the help of which the appearance of an impurity in the gaseous carrier can be determined. A schematic diagram of a gas chromatograph is shown in Fig. 23.
If detector readings are recorded as a function of time, a chromatogram is obtained from a series of peaks, each of which corresponds to one of the components of the mixture.

Rice.
/ - cylinder with carrier gas; 2,4,6 - thermostats; 3 - dispenser; 5 - column; 7 - detector; 8 - registrar; 9 - computing integrator
The intensity of each peak, or more precisely its area, is a measure of the concentration of each of the components passing through the detector (Fig. 24).
In analytical practice, the GLC method is used much more widely than GAC. It is extremely widely used in the oil, gas and coke-chemical industries for the separation and analysis of multicomponent mixtures of organic substances, waste gases (including sulfur-, nitrogen- and halogen-containing), for the analysis of gases formed during the development of sulfide raw materials and during the recovery of ferrochemicals -

Rice. 24. Chromatogram of a complex gas mixture of ronickel. It is also used in the analysis of pharmaceutical and perfumery products, herbicides and pesticides, in biochemical analysis, in the study of various natural products of plant origin, etc. The GLC method can be used for the analysis of inorganic substances after their preliminary conversion into the form of suitable gaseous or volatile compounds . These are, for example, chlorides of some metals (T1CI4, SbCh, etc.), tetramethyl derivatives of silicon, germanium, tin, lead, beryllium, aluminum, chromium acetylacetonates, boranes (hydrogen borides), phosphorus compounds, etc. The advantages of the method include a very small amount of substance for analysis: in the case of liquid samples ~0.001-0.0 K) ml, for gases - 1-10 ml.
High sensitivity and separation ability, reliability, good reproducibility of results, speed of analysis, and the possibility of using GLC for the analysis of mixtures of complex substances in a stream have led to widespread use of gas chromatographs to automate the control of production processes. The sensor of an industrial chromatograph is used not only as a recording device, but also as a control device that supplies signals directly to the actuators. Thus, an industrial chromatograph can monitor and regulate the most important parameters of the technological process - temperature, pressure, raw material flow, etc.
Summarizing all of the above about chromatographic methods, it should be noted that they are especially valuable as an effective tool for separating compounds with similar chemical properties, even isotopes, since minor differences in composition or structure are usually sufficient to cause a noticeable difference in the ability of components to be retained on one or another other sorbents. It is also important that during separation the substances do not undergo chemical changes and are released in the form in which they were present in the original mixture.
Chromatographic methods are characterized by simplicity of experiment, selectivity and versatility, i.e. the ability to be used for the separation and determination of liquid and gaseous inorganic and organic compounds in a wide range of concentrations. The main disadvantage of these methods is that they take a long time, especially when separating substances with similar properties. However, the creation of special devices - high-pressure liquid chromatographs, in which the eluent is fed into the column at a speed 100 times greater than in conventional column chromatography, and under pressure up to 0.5-40 MPa - allows, in favorable cases, to completely separate 20 or 30 components samples within a few minutes. The detection limit, usually determined by the sensitivity of the detector, is 10 -3 -10 -6%, with a sample weight of 1-10 mg, the error is 0.2-2%. This option is called high-performance liquid chromatography (HPLC).
The possibility of automating the process of separating components in combination with physical methods for their determination (in particular, mass spectral) led to the use of chromatography for monitoring and automatic regulation of technological processes. Thanks to the noted features, chromatographic methods have become most widespread as methods for separating complex mixtures of substances and as methods for concentrating microimpurities.
- Katin is a highly dispersed plastic rock consisting of the mineral kaolinite Al2O3.2SiO2.2H2O and various impurities (quartz, mica, feldspar, etc. Trepel is one of the natural hydrated forms of silica.
The problem of water purification has long worried humanity. Today there are many ways to cleanse it. One of the most common, without a doubt, is sorption water purification. What is its essence?
From this article you will learn:
What is sorption water purification
How does sorption water purification occur?
What filters are used for sorption water purification
What types of sorbents are used
Sorptive water purification - what is it?
Sorption water purification is a highly effective method of deep purification, in which the effect is achieved by binding particles of chemicals and various impurities at the molecular level. Such water purification allows you to remove even organic compounds that cannot be separated by any other methods.
Modern highly active sorbents work effectively in water with any, even the smallest, concentration of unwanted impurities. As a result of sorption purification, there is practically no residual concentrate in the water.
The term “adsorption” means the absorption of a substance from a gaseous medium or solution by the surface layer of another substance. This process also occurs in the water that we purify when certain substances are added. The adsorbent attracts molecules of unwanted impurities to its surface and never releases them.
Sorption purification is especially effective at the final stage of a high level of purification, when water, having passed through the previous stages of purification, has left almost everything unnecessary on the filters, and now it is necessary to remove the smallest concentrations of unwanted impurities.
How quickly and efficiently this process will take place depends on the following factors:
sorbent structures;
temperature at which the process takes place;
type and concentration of harmful substances in water;
environmental reaction activity.
Why is sorption water purification needed and where is it used?
Sorption water purification has been known to people for quite some time. Both earlier and to this day, for example, they used carbon filtration, which works well in closed systems, deeply cleaning, including from organic matter.
Sorption water purification gives excellent results, removing various organic substances, making it possible to clean wastewater from dyes or other hydrophobic compounds. This method has also gained wide popularity due to the fact that it does not require significant material costs.
Sorption water purification can be used as an independent method, or can be used in combination with biological purification. However, this method cannot be used when contaminated only with inorganic impurities or organic impurities of low molecular weight. It purifies water not only from impurities, which can only be seen in the results of laboratory tests, but also from foreign odors and the taste of chlorine and hydrogen sulfide that are easily detected by humans.
Activated carbon is an effective adsorbent that has micropores in its structure that successfully perform filtration. It is not difficult to obtain: the raw materials for production are wood, peat, nut shells, and animal products. Applying silver ions to the surface of activated carbon particles extends the service life of the adsorbent, preventing it from being damaged by microbes.
Activated carbon is one of the best sorbents used in modern systems today. It comes in different types. The highest quality of sorption water purification will be provided by the one that has the maximum possible number of micropores.
Sorptive water purification with activated carbon is usually used to remove organic matter from water during its preparation before reverse osmosis. At the same time, the liquid is also cleared of chlorine, which makes it more acceptable for hygiene procedures.

Filters filled with activated carbon can become unusable if colloidal particles enter them with water, which prevent micropores from performing their functions. In this case, you have to change the sorbent or restore it.
Sorptive water purification using activated carbon filters significantly improves the quality of the liquid, freeing it not only from chlorine, but also from nitrogenous compounds. With simultaneous sorption and ozonation of water, the capabilities of activated carbon to purify it from impurities are significantly increased. If natural minerals with the addition of Ca, Mg and aluminum oxides are used as a sorbent, the water is purified from phosphorus compounds.
Sorption filters do an excellent job of purifying water from iron when insoluble oxides in the form of solid particles are formed in the liquid after the oxidation process.
What types of sorption water purification are divided into?
The type of sorption purification process is:
periodic;
continuous.
According to the hydrodynamic regime there are:
displacement installations;
mixing installations;
intermediate type installations.
Depending on the state of the layers of the sorbent used, cleaning can be:
moving;
motionless.
According to the direction of filtration, cleaning occurs:
counterflow;
direct flow;
mixed traffic.
Based on the contact of interacting phases, the cleaning process is divided into:
stepped;
continuous.
According to the design of the filter, cleaning can be:
column;
capacitive
Main types of sorbents
We have already said that a very popular type of sorbent for water purification is activated carbon, which perfectly removes organic compounds of natural and artificial origin. But, in addition to activated carbon, other types of sorbent are also used.
Carbon-free sorbents for water purification
The most widely used sorption water purification technology is purification using carbon-free sorbents. They can be of either natural or artificial origin: clay rocks, zeolites, etc.
Non-carbon sorbents have a number of advantages, such as:
increased capacity;
ability to exchange cations;
prevalence and, accordingly, low price.
Clay rocks

Clay rocks often play the role of a water filter in nature. The ability of this material is successfully used for the same purposes by humans. Such rocks have layered rigidity, a well-developed structure with a large number of micropores of various sizes.
Sorptive water purification using filters, where clay rocks act as a sorbent, is a complex process that includes van der Waals reactions. As a result, the water becomes crystal clear in appearance and is freed from toxic organic compounds of chlorine, herbicides, and surfactants.
Clay rocks are also convenient because they are accessible to mining. This increases their consumption.
Zeolites
Zeolites are a group of minerals with a characteristic glassy luster. Today natural and artificial zeolites are used. They have an interesting structure: a three-dimensional aluminosilicate frame with a regular tetrahedral structure and a negative charge. Hydrated ions of alkali and alkaline earth metals are located in the voids of the framework and have a positive charge that compensates for the charge of the framework. Zeolites are called a sieve for molecules, since they trap substances whose molecules are smaller than the voids of the framework.
More than 30 types of zeolites are known. The most used ones, which are easy to mine and process: abasite, mordenite, clinoptilolite.

Before using zeolite as a sorbent, it is calcined with sodium chloride carbonate in an oven at a temperature of +1000 ° C, after which organosilicon compounds are formed on its surface, giving it hydrophobic properties.
Zeolites are used for sorption purification of water in powder form. They purify water from:
organic compounds.
colloidal and bacterial contaminants;
pesticides;
dyes;
Inorganic ion exchangers
Most of them are used in the form of salts, since they cannot exist in the hydrogen form. But this does not allow water to be desalted without the participation of rare anion exchangers of inorganic minerals. Therefore, it is necessary to use organic cation exchangers and anion exchangers based on synthetic organics.
Organic ion exchangers

Many organic ion exchangers have a gel structure. They do not have pores, but in an aqueous solution they swell and can exchange ions.
There are macroporous ion exchangers that work like activated carbon, which are less capacious than gel ones, but have improved exchange and sieve effect, are resistant to mechanical load, and are osmotically stable.
An important achievement of our time has been the possibility of synthesizing organic ion exchangers with specified properties that are not found in nature.
What does a sorption filter for water purification consist of?
Each sorption filter has the following parts:
a body of a certain size in the form of a fiberglass cylinder;
a stationary layer of activated carbon with a gravel backing;
a control valve of a certain type (sometimes a mechanical valve);
pipeline for supplying waste water;
pipeline for removing purified water;
pipeline for supplying loosening water;
drainage and distribution system.
The speed of the filter directly depends on how contaminated the water is to be purified. The grain size of the sorbent also influences (from 1 to 5 mm). The linear rate of water filtration can vary from 1 to 10 m 3 /hour.

The best way to purify water by sorption is to feed it into the filter from the bottom up, when the entire cross-sectional area of the filter is filled with water evenly, and bubbles easily come out of the water.
For regenerative wastewater treatment with subsequent recycling of retained valuable elements in a sorption filter, filters with a fixed sorbent layer are used for water purification. You can later extract the necessary elements using water vapor or chemical solvents.
You can study in detail the operation of a sorption water purification system using the FSB series filter as an example. This model is designed to work in storm sewer systems. At the entrance to the filter there are pre-filters: a sand catcher and an oil catcher, the task of which is not to miss the first strong contaminants that can quickly damage the filter.
Having passed through the pre-filters, the water enters the sorption block through the pipe, from where the distribution and discharge pipe discharges the water to the lower distribution zone.
When water hits the sorbent located here, it is evenly distributed over it and passes through, being cleaned of impurities. Moreover, the brand and volume of sorbent used is selected depending on the initial and final concentration levels of harmful substances and the required productivity.
Purified water is directed by an ascending flow into a collecting circular tray and discharged through a pipe.
Procedure for installing the system:
Dig a pit of the required size.
Fill its bottom with a 300 mm layer of sand and compact it well.
On a sand bed, pour a reinforced concrete slab with a thickness of at least 300 mm, the dimensions of which are 1000 mm wider than the diameter of the filter housing.
Mount the body of the sorption unit on the stove, carefully maintaining its verticality.
To ensure that the housing is stable, first fill it with water (to the level of the perforated bottom).
Secure the body with anchors so that it does not move when backfilling.
Fill the pit with clean sand to the level of the inlet and outlet pipes. This must be done in stages, in layers of 300 mm, carefully compacting each layer.
Connect the inlet, outlet and overflow pipelines. Next, fill the body with sand to the top, carefully compacting the soil so as not to damage the installed pipes.
Fill the body with filler from bags, constantly carefully distributing it over the entire area of the bottom.
Thoroughly rinse the installed sorbent before putting the system into operation.
Finally, the housing must be filled with clean water.
If your sorption water purification system must purify water from all possible types of pollution, then it is necessary to use a complex of sorbents: activated carbon and various ion exchange substances, which must be selected taking into account the impurities found in the water of your source.
There are many types of sorption water purification systems. To choose the one that is right for you, study all the factors and conduct laboratory tests of the water. Installing water purification equipment also requires special knowledge. Therefore, it should be performed by professionals.
There are many companies on the Russian market that develop water treatment systems. It is quite difficult to choose one or another type of water filter on your own, without the help of a professional. And even more so, you should not try to install a water treatment system yourself, even if you have read several articles on the Internet and it seems to you that you have figured it out.
It is safer to contact a filter installation company that provides a full range of services - specialist consultation, analysis of water from a well or well, selection of suitable equipment, delivery and connection of the system. In addition, it is important that the company provides filter maintenance.
By collaborating with Biokit, you get the widest selection of reverse osmosis systems, water filters and many other devices designed to purify water and return it to its natural qualities.
We are ready to help you in these areas:
Choose a water filter.
Connect the filtration system.
Select replacement materials.
Troubleshoot equipment problems.
Involve specialist installers.
Provide telephone consultation on questions of interest.
Entrust water purification to Biokit professionals who care about your health.